Introduction
Osteoarthritis (OA) is clinically the most common form of arthritis, associated with articular cartilage damage and degradation [1]. OA aetiologies include abnormal loading, repetitive injuries, ageing, and obesity [2]; OA increases socioeconomic burdens worldwide, including China [3]. OA is associated with physical disabilities and joint pain. Several degradative pathological changes are evident in cartilage [4]; and chondrocytes undergo apoptosis [5]. Both osteoblasts and chondrocytes are formed via differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) [6]. Such cells are used to manage olfactory disorders, pelvic floor dysfunction, and pulmonary injury caused by ischaemia. BMSCs regenerate cartilage in animal models of OA; the cells are simple to isolate, multipotent, and highly proliferative [7]. BMSCs ameliorate OA-induced cartilage injury by differentiating into chondrocytes.
miRNA dysregulation is linked to the development of several diseases, including OA, and the miRNAs may serve as useful therapeutic targets [8]. miRNA dysregulation induces the production of inflammatory mediators, remodelling of the extracellular matrix, and dysregulation of bone formation [9]. miR-223 expression slows the cartilage degeneration of OA and rheumatoid arthritis [10]. Several molecules ameliorate cartilage degeneration by enhancing miR-223 expression and reducing that of NLRP3 [11], thus lowering inflammatory cytokine levels [12]. NLRP3 expression is downregulated by miR-223-3p; we thus evaluated the effect of miR-223-3p on the chondrocytic differentiation of BMSCs.
Material and methods
Animals
Sprague-Dawley rats (200–250 g) were housed and maintained as per international guidelines; the animal study was approved by the Animal Ethics Committee of Beijing Hospital, China (no. IAEC/BH/2018/09).
Chemicals
The miR-223 inhibitor and mimic were procured from Invitrogen (USA). The ELISA kits and primers were the products of Cell Signaling Technology Inc. (USA).
Chondrocyte isolation and culture
Primary BMSCs were isolated as described previously [13] from the cavities of the tibial plateaus and femora and cultured for 2 days at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with transforming growth factor-β 3 (TGF-β), sodium pyruvate, ascorbate, and dexamethasone. Non-adherent cells were removed during medium changes, which were performed until the cells attained 80–90% confluence. Flow cytometry was used to characterise the BMSCs in terms of typical surface markers. Antibodies against CD29, CD90, CD45, or CD105 were added to BMSCs following the manufacturer’s directions.
Alcian blue staining
BMSCs were cultured for 0, 14, and 21 days in chondrogenic or normal medium and fixed in 4% (v/v) formalin at 4°C; Alcian blue 8GX (1% v/v) was added and staining proceeded for 60 min at room temperature. The cells were washed with water and a flatbed scanner was used to detect signals. Staining intensity was quantified with the aid of Image-Pro Plus software.
RT-PCR
Trizol reagent was used to extract total RNAs, followed by reverse transcription into cDNAs. The levels of miR-223 and β-actin expression in BMSCs were derived with the aid of the SYBR Green gene expression assay. miR-223 levels were also analysed using a TaqMan microRNA assay kit (Table I).
Cell transfection
Isolated cells were seeded into 12-well plates and incubated for 1 day at 37°C in medium with Lipofectamine 2000. The miR-223 inhibitor or mimic was added, and incubation continued for a further day. Control cells were not transfected.
In-vivo model of osteoarthritis
The animals were divided into normal, OA, and miR-223 mimic-treated groups. All were injected with 7 mg/kg xylazine and 60 mg/kg of ketamine. To induce OA, the medial side of the patellar tendon was incised, the patella dislocated, and the anterior cruciate ligament transected with a surgical blade. The medial retinaculum was repaired, and the skin closed. The miR-223 mimic was delivered directly to the articular cavity. qRT-PCR was performed (as mentioned above).
Estimation of protein and cytokine levels
All animals were sacrificed via decapitation and articular cartilage isolated from the medial tibial plateaus. qRT-PCR was used to quantify the levels of mRNAs encoding NLRP-3, IL-18, MMP-3, and Col II in tissue homogenates. IL-1β, IL-6, IL-18, TGF-β, and NF-kB levels were measured by ELISA (Table II).
Table II
The primers
Histopathological analyses
Isolated knee joints were fixed in 10% (v/v) formalin for 3 days, placed into molten paraffin, and cut into 6 µm-thick sections when the paraffin set. The sections were stained with Safranin-O and cartilage degeneration scored as described previously.
Immunohistochemical analysis
The subchondral bone was sectioned after embedding in paraffin wax. Ethanol baths were used to dehydrate the sections, which were then incubated with an anti-rat NLRP3 antibody at 4°C overnight. Biotinylated IgG was added and incubation continued for 30 min at 37°C. The sections were then stained with diaminobenzidine (DAB) and cells counted under an optical microscope.
Statistical analysis
All data are expressed as means ± standard errors of the means (SEMs). Statistical analysis featured one-way analysis of variance (ANOVA). Post-hoc comparisons were performed using the Dunnett test of GraphPad Prism ver. 6.1 software (USA). A p-value < 0.05 was taken to indicate statistical significance.
Results
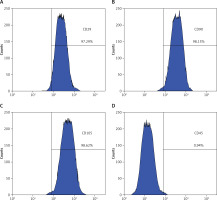
BMSC characterisation
BMSCs were assessed via flow cytometry (Figures 1 A–D). The cells were CD29-, CD90-, and CD105-positive and CD45-negative, indicating that they were BMSCs.
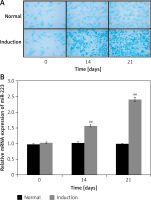
miR-223 expression by BMSCs
miR-223 expression during BMSC chondrogenic differentiation was assessed as shown in Figures 2 A and B. The extent of BMSC differentiation on days 0, 14, and 21 was evaluated by staining glycosaminoglycan with Alcian blue (Figure 2 A). Staining increased significantly by 14 and 21 days when cells were cultured in chondrogenic compared to normal medium. qRT-PCR revealed that miR-223 expression was upregulated in cells growing in chondrogenic medium (compared to normal medium) on days 14 and 21.
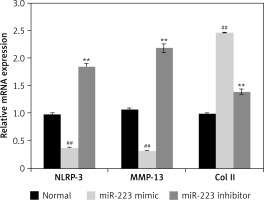
Effects of miR-223 mimic and inhibitor on expression levels of NLRP-3, MMP-13, and Col II
We used Western blotting to measure the expression levels of NLRP-3, MMP-13, and Col II in chondrogenically differentiated BMSCs treated with the miR-223 mimic and the inhibitor (Figure 3). The miR-223 mimic significantly (p < 0.01) downregulated the expression of NLRP-3, MMP-13, and Col II, compared to controls. The miR-223 inhibitor significantly (p < 0.01) upregulated the expression levels of all three proteins.
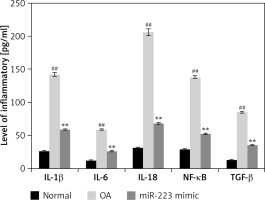
Effects of miR-223 mimic on levels of inflammatory cytokines
The levels of IL-1β, IL-6, IL-18, NF-κB, and TGF-β were determined in miR-223 mimic-treated cartilage of rats with OA (Figure 4). The levels were higher (p < 0.01) in rats with OA than normal rats. Treatment with the miR-223 mimic reduced (p < 0.01) the cytokine levels.
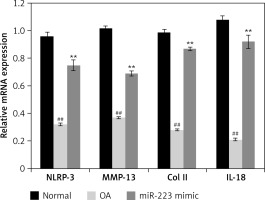
Effects of miR-223 mimic on expression of mRNAs encoding NLRP-3, IL-18, MMP-3, and Col II
Figure 5 shows the effects of the miR-223 mimic on the levels of mRNAs encoding NLRP-3, IL-18, MMP-3, and Col II in cartilage of rats with OA. All mRNAs were expressed at lower levels than in normal rats. Treatment with the miR-223 mimic significantly (p < 0.01) enhanced the expression levels of all tested mRNAs.
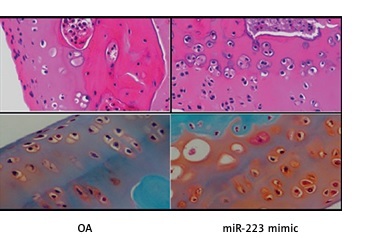
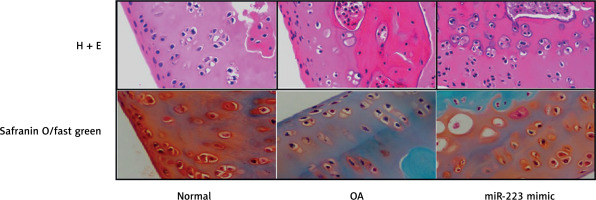
Effect of miR-223 mimic on histopathology of cartilage tissue
We stained the cartilage of miR-223 mimic-treated rats with OA with H&E and safranin O/Fast Green (Figure 6). Cartilage erosion was more severe in the OA than the normal group. The miR-223 mimic attenuated erosion.
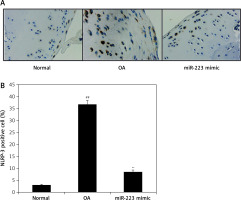
Effect of miR-223 mimic on NLRP-3 expression
We immunohistochemically explored the effect of the miR-223 mimic on NLRP-3-positive cell numbers in the cartilage of rats with OA (Figure 7). The numbers were significantly higher (p < 0.01) than in normal rats. The miR-223 mimic reduced the proportion of NLRP-3-positive cells in cartilage (p < 0.01).
Discussion
Osteoarthritis is a major form of arthritis. Several drugs are available, but chronic OA requires chronic treatment. The drugs have several limitations; new therapies are required. miR-223 is expressed in healthy chondrocytes but the levels fall in degenerating chondrocytes. miR-223 controls NLRP-3 expression and thus, in turn, the levels of cytokines that contribute to the chondrocyte destruction characteristic of OA. We explored whether miR-223 protected against OA.
In OA, chondrocytes are damaged by foreign body intrusions, inflammation, and excessive joint loading [14]. Chondrocytes do not self-heal or regenerate, and proliferation is inhibited by the reduced cell density [15]. Management focuses on cartilage engineering; regeneration is inefficient [16]. BMSCs differentiate into myocytes, osteoblasts, and chondrocytes [17]. Several genes are involved in chondrogenesis. miR-223 enhanced the chondrogenic differentiation of BMSCs [18] and alleviated OA chondrocyte destruction [19]. We found that miR-223 enhanced the chondrogenic differentiation of BMSCs.
NLRP-3, MMP-13, and Col II facilitate BMSC differentiation [20] toward chondrocytes [21]. In OA, the MMP-13 level is reduced and that of Col II enhanced in damaged chondrocytes [22]. MMP-13 first enhances and then reduces Col-II expression in chondrogenically differentiated BMSCs [23]. miR-223 increased NLRP-3 expression in such cells. The miR-223 mimic reduced NLRP-3 expression by BMSCs.
Cartilage destruction in OA is attributable to increased cytokine levels (including that of IL-18) [24] caused by a reduced level of NLRP-3. The cytokines reduce the expression levels of MMP-13, Col II, and TGF-β, all of which stimulate chondrocyte differentiation [25, 26]. The miR-223 mimic reduced cytokine expression, and increased the levels of NLRP-3, MMP-13, Col II and TGF-β, in the cartilage of rats with OA.
In conclusion, we found that the miR-223 mimic enhanced the chondrogenic differentiation of BMSCs by regulating the NLRP-3/IL-18/TGF-β pathway. The mimic may thus usefully manage OA.