Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
HEPATOLOGY / CLINICAL RESEARCH
miR-212-3p targets nuclear factor I A (NFIA) to suppress hepatitis B virus replication and tumor progression in hepatocellular carcinoma via repressing enhancer I activity
1
Department of Infectious Disease, Huaihe Hospital of Henan University, Kaifeng, China
Submission date: 2019-07-23
Final revision date: 2019-09-19
Acceptance date: 2019-09-30
Online publication date: 2021-03-25
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Emerging evidence identifies that microRNAs (miRNAs) are associated with hepatitis B virus (HBV) infection. In the current study, we mainly focus on the functions and underlying mechanisms of miR-212-3p in HBV replication in hepatocellular carcinoma (HCC).
Material and methods:
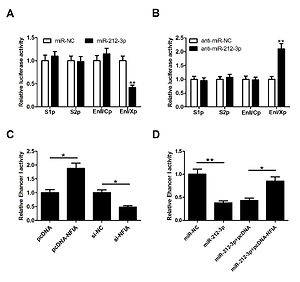
The levels of miR-212-3p, nuclear factor I A (NFIA) and HBV DNA copies were measured by qRT-PCR. The level of core particle-associated HBV DNA, the production of hepatitis B surface antigen (HBsAg) and hepatitis B e-antigen (HBeAg), and the expression of NFIA were detected via southern blot assay, ELISA and western blot assay, respectively. The putative target of miR-212-3p was predicted by TargetScan and Pictar, followed by the dual luciferase reporter assay and RNA immunoprecipitation (RIP) assay to validate the interaction. The interaction between miR-212-3p and enhancer I/X promoter (EnI/Xp) reporter was also verified by dual luciferase reporter assay. In addition, the cell viability and apoptotic rate were detected by MTT and flow cytometry, respectively.
Results:
miR-212-3p mimics or NFIA knockdown inhibited HBV expression and replication in HepG2.2.15 cells, while miR-212-3p inhibitor or NFIA overexpression showed the opposite trend. NFIA was confirmed as a direct target of miR-212-3p. Furthermore, miR-212-3p impeded HBV expression and replication by suppressing NFIA. Also, miR-212-3p lowered EnI/Xp activity by regulating NFIA. In addition, miR-212-3p retarded cell viability and induced apoptosis through targeting NFIA.
Conclusions:
miR-212-3p targets NFIA to down-regulate its expression, thereby inhibiting HBV replication and tumorigenesis in HCC. Our findings might provide a promising therapeutic target for HBV infection.
Emerging evidence identifies that microRNAs (miRNAs) are associated with hepatitis B virus (HBV) infection. In the current study, we mainly focus on the functions and underlying mechanisms of miR-212-3p in HBV replication in hepatocellular carcinoma (HCC).
Material and methods:
The levels of miR-212-3p, nuclear factor I A (NFIA) and HBV DNA copies were measured by qRT-PCR. The level of core particle-associated HBV DNA, the production of hepatitis B surface antigen (HBsAg) and hepatitis B e-antigen (HBeAg), and the expression of NFIA were detected via southern blot assay, ELISA and western blot assay, respectively. The putative target of miR-212-3p was predicted by TargetScan and Pictar, followed by the dual luciferase reporter assay and RNA immunoprecipitation (RIP) assay to validate the interaction. The interaction between miR-212-3p and enhancer I/X promoter (EnI/Xp) reporter was also verified by dual luciferase reporter assay. In addition, the cell viability and apoptotic rate were detected by MTT and flow cytometry, respectively.
Results:
miR-212-3p mimics or NFIA knockdown inhibited HBV expression and replication in HepG2.2.15 cells, while miR-212-3p inhibitor or NFIA overexpression showed the opposite trend. NFIA was confirmed as a direct target of miR-212-3p. Furthermore, miR-212-3p impeded HBV expression and replication by suppressing NFIA. Also, miR-212-3p lowered EnI/Xp activity by regulating NFIA. In addition, miR-212-3p retarded cell viability and induced apoptosis through targeting NFIA.
Conclusions:
miR-212-3p targets NFIA to down-regulate its expression, thereby inhibiting HBV replication and tumorigenesis in HCC. Our findings might provide a promising therapeutic target for HBV infection.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.