Introduction
Chondrocyte apoptosis, known as programmed cell death, as a normal physiological phenomenon is responsible for modulating the homeostasis of articular cartilage, while excessive apoptosis of chondrocytes is a central pathological incident in articular cartilage injury or osteoarthritis (OA) [1, 2]. A substantial body of evidence reveals that mechanical overload and inflammatory cytokines are the primary factors contributing to cartilage degradation and chondrocyte apoptosis [3, 4]. Investigation into the molecular mechanisms of apoptosis of chondrocytes has revealed multiple signaling pathways, including tumor necrosis factor-α (TNF-α)/TNF receptors (TNFR), caspase, Fas/FasL, p53 and myc, participating in this pathological process [5–9].
TNF receptor superfamily member 1A-associated death domain (TRADD), as a novel 34-kDa protein, specifically interacts with TNFR to activate apoptosis or necroptosis via nuclear factor-κB (NF-κB) activation and caspase-8 (casp-8)/casp-3 [10]. Over-activation of TRADD signaling is implicated in TNF-α-induced apoptosis and necrosis in many cells, including osteoclasts, neuronal cells, keratinocytes and Jurkat T cells [10–13]. Moreover, up-regulation of TRADD mRNA or protein expression is involved in ofloxacin- or interleukin-1β-induced apoptosis of chondrocytes [5, 14]. However, the roles of TRADD in TNF-α-caused chondrocyte apoptosis are still unclear.
MicroRNAs (miRNAs) have been validated as a novel class of small non-coding RNAs with the length of 18-25 nucleotides, which are implicated in expression regulation of more than one-third of human genes via post-transcriptional repression [15, 16]. Numerous studies indicate that miRNAs participate in a variety of biological processes, such as proliferation, differentiation and apoptosis [17, 18]. In addition, miRNAs have also been demonstrated to modulate disease progression, including cancer, diabetes mellitus, cardiovascular diseases, acute gouty arthritis and OA [18–20]. Several differentially expressed miRNAs have been identified to be related to chondrocyte apoptosis [21, 22]. However, the exact etiological mechanism of miRNA in the pathogenesis of TNF-α-induced chondrocyte apoptosis is not clear. In the present study, we aimed to explore whether TRADD-specific miRNAs participate in the regulation of chondrocyte apoptosis. The results demonstrated that a total of 13 miRNAs were filtered by both TargetScan and miRDB. Among these miRNAs, we found that miR-149-5p as a candidate miRNA was significantly repressed in TNF-α-stimulated chondrocyte. Furthermore, miR-149-5p mimics were transfected into chondrocytes to investigate the effect of miR-149-5p gain-of-function in TNF-α-induced chondrocyte apoptosis in vitro.
Material and methods
Cell culture
Human primary chondrocytes were obtained as described previously [2] and were cultured in DMEM-F12 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 5% CO2 and 95% air as described previously [2].
Cell counting kit 8 (CCK8) assay
Cell viability was detected using a CCK-8 kit (Beyotime Institute of Biotechnology, Haimen, China). Absorbance was recorded at 450 nm.
Enzyme linked immunosorbent assay (ELISA)
The levels of IL-1β (Cat. no: E-EL-H0149c), IL-6 (Cat. no: E-EL-H0102c) and IL-18 (Cat. no: E-EL-H0253c) in the supernatant of cultured chondrocytes were measured using ELISA kits (Elabscience Biotechnology Co., Ltd, Wuhan, China) as described previously [23].
Annexin V-FITC double staining for apoptosis analysis
Annexin V-FITC kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to evaluate the apoptotic cell proportion as described previously [23].
miR prediction
The bioinformatics algorithm was executed by TargetScan (www.targetscan.org) and miRDB (www.mirdb.org) to predict the potential miRNAs which could target modulation of TRADD expression via binding with its 3′-untranslated regions (3′-UTRs).
Cell transfection and plasmid constructs
miR-control (miR-Con; 5′-UACUUAGUCCGGGAGUCCCAGUC-3′) and miR-149-5p mimics (5′-UCUGGCUCCGUGUCUUCACUCCC-3′) were synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China) and were transfected into chondrocytes using Lipofectamine 2000 (Invitrogen) as described previously [24]. A mammalian expression plasmid designed to specially express the full-length open reading frame of human TRADD without 3′-UTR, which did not contain the conserved complementary sequence binding with miR-149-5p, was purchased from GeneCopoeia, Inc. (Rockville, MD, USA). An empty plasmid served as the negative control. Vector-Con and vector-TRADD were transfected into chondrocytes using Lipofectamine 2000 (Invitrogen) as described previously [24].
Luciferase reporter assay
The wild-type (WT) or mutant-type (Mut) 3′-UTR of TRADD was inserted into the multiple cloning sites of the luciferase expressing pMIR-REPORT vector (Ambion; Thermo Fisher Scientific, Inc.). For the luciferase assay, chondrocytes containing the WT or Mut 3′-UTR of TRADD (0.5 µg) were co-transfected with miR-Con or miR-149-5p mimics using Lipofectamine 2000, according to the manufacturer’s protocols. The luciferase activity was measured using a luciferase reporter assay kit (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocols.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
miRNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA) was used to extract total RNA. TaqMan RT kit and TaqMan MicroRNA assay (Applied Biosystems) were used to detect miRNA expression levels using the Applied Biosystems 7300 Real-Time PCR system, according to the manufacturer’s protocol. U6 small nuclear RNA was used as an endogenous control. The relative expression levels of miRNAs were calculated using the 2-ΔΔCq method [25]. The primers of miRs for qPCR are shown in Table I.
Table I
miRs primers for RT-qPCR
Western blotting
Western blotting were performed as previously described [26]. Membranes were cultured with primary antibodies of TNFR1 (Cat. no: sc-8436; dilution: 1 : 1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), TRADD (Cat. no: sc-46653; dilution: 1 : 1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), cleaved caspase-3 (Cat. no: 9661; dilution: 1 : 1,000; Cell Signaling Technology, Beverly, MA, USA) and cleaved caspase-8 (Cat. no: 9748; dilution: 1 : 1,000; Cell Signaling Technology, Beverly, MA, USA) at room temperature for 2 h. Then the membranes were incubated with an appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 2 h and visualized with an enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.). Signals were analyzed with Quantity One software version 4.5 (Bio Rad Laboratories, Inc., Hercules, CA, USA). Anti-β-actin (Cat. no: sc-47778; dilution: 1 : 2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used as the control antibody.
Ethics
The present study was approved by the First Affiliated Hospital of Harbin Medical University (Harbin, China).
Statistical analysis
Data were presented as the mean ± standard error of the mean. Statistical analysis was performed using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism Version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Student’s t-test was used to analyze the differences between the two groups. Differences between multiple groups were analyzed by one-way analysis of variance, followed by a post-hoc Tukey test. P < 0.05 was considered to indicate a statistically significant difference.
Results
TNF-α inhibited chondrocyte growth and elevated the production of inflammatory cytokines and apoptotic cell proportion
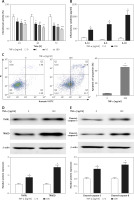
Chondrocytes were exposed to TNF-α for various durations at different concentrations, the CCK8 assays demonstrated that TNF-α stimulation led to significant cell growth inhibition in a time- and concentration-dependent manner (Figure 1 A). The 50% inhibition rate of TNF-α on chondrocyte growth occurred after TNF-α treatment at the concentration of 100 ng/ml for 48 h (Figure 1 A). In addition, the levels of inflammatory cytokines, including IL-1β, IL-6 and IL-18, were significantly higher in TNF-α-treated chondrocytes than those of the control group (Figure 1 B). We also found that the apoptotic cell proportion was increased in chondrocytes in the presence of TNF-α treatment (Figure 1 C). Western blotting analysis revealed that cell apoptosis-related signaling pathways, TNFR1/TRADD and caspase-3/8, were activated in TNF-α-treated chondrocytes (Figures 1 D, E).
Figure 1
TNF-α induced chondrocyte damage. A – CCK8 assays were performed to detect cell viability with or without TNF-α (100 ng/ml) treatment. B – ELISA assays were used to measure inflammatory cytokines. C – TNF-α-induced apoptosis in chondrocytes was analyzed by flow cytometry. D, E – Protein expression was measured by western blotting. n = 3 in each group, *p < 0.05 compared with control group
Expression of post-transcriptional regulators-miRNAs
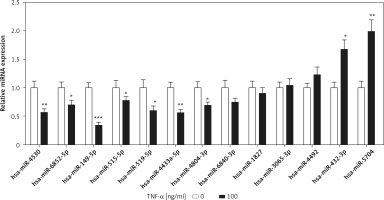
miRNAs as post-transcriptional regulators have been reported in the process of chondrocyte apoptosis [3]. However, TRADD-related miRNAs have not been described in TNF-α-induced chondrocyte apoptosis. We used the bioinformatics algorithms TargetScan (www.targetscan.org) and miRDB (www.mirdb.org) to predict TRADD-related miRNAs, and the results showed that a total of 13 miRNAs were filtered by both TargetScan and miRDB. Among these miRNAs, 7 miRNAs and 2 miRNAs were significantly down-regulated and up-regulated, respectively, in TNF-α-treated chondrocytes compared with those of the control group. Otherwise, 4 miRNAs showed no significant difference between the two groups (Figure 2). According to the |log2 (fold change)|, miR-149-5p was the maximum value. Therefore, we focused on miR-149-5p in our further experiments.
Figure 2
Expression of TRADD-associated miRNAs. Bioinformatics algorithms, TargetScan and miRDB, were used to predict TRADD-related miRNAs, and the expression of 13 miRNAs was measured using RT-qPCR in chondrocytes with or without TNF-α (100 ng/ml) treatment. n = 3 in each group, *p < 0.05 compared with control group
miR-149-5p directly targeted TRADD
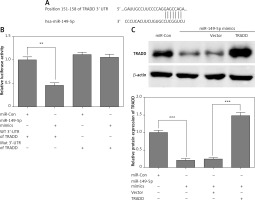
First, we found a conserved complementary sequence between the 3′-UTR of TRADD and miR-149-5p, which was predicted by TargetScan (Figure 3 A). As shown in Figure 3B, miR-149-5p mimics reduced luciferase activity significantly compared with the control group, while the luciferase activity showed no obvious change in chondrocytes co-transfected with the Mut 3′-UTR of TRADD and miR-149-5p mimics. Western blotting analysis indicated that miR-149-5p mimics significantly attenuated the expression of TRADD compared with the control group (Figure 3 C). Meanwhile, we performed a rescue experiment in miR-149-5p mimic transfected chondrocytes by transfecting TRADD overexpressed plasmids, which did not contain the 3′-UTR of TRADD. Therefore, they were not targeted by miR-149-5p. As shown in Figure 3 C, miR-149-5p mimics induced the down-regulation of TRADD protein expression, which was reversed by the transfection of TRADD plasmids.
Figure 3
miR-149-5p directly targeted TRADD. A – The conserved complementary sequence between the 3′-UTR of TRADD and miR-149-5p was predicted by TargetScan. B – Luciferase activity was measured in chondrocytes after transfection with WT or Mut 3′-UTR of TRADD combined with miR-149-5p mimics or miR-Con. C – After transfection with miR-149-5p mimics or miR-149-5p mimics combined with TRADD vector into chondrocytes, the protein expression of TRADD was measured by western blotting. n = 3 in each group, **p < 0.01 and ***p < 0.001
miR-149-5p mimics inhibited TNF-α-induced growth inhibition, inflammation and apoptosis in chondrocytes, which were reversed by overexpression of TRADD
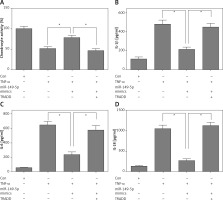
After treatment with chondrocytes by TNF-α (100 ng/ml) for 48 h, CCK8 assays showed that overexpression of miR-149-5p alleviated TNF-α-caused chondrocyte growth inhibition (Figure 4 A). Moreover, the TNF-α-induced increase in the production of inflammatory cytokines, including IL-1β, IL-6 and IL-18, was also attenuated by overexpression of miR-149-5p (Figures 4 B–D). Furthermore, an elevation of apoptotic cell proportion in TNF-α-treated chondrocytes was partially mitigated by miR-149-5p mimics (Figures 5 A, B). However, the protective effects of miR-149-5p mimics on TNF-α-induced growth inhibition, inflammation and apoptosis in chondrocytes were reversed by overexpression of TRADD (Figures 4 A–D, 5 A, B).
Figure 4
miR-149-5p mimics inhibited TNF-α-induced growth inhibition and inflammation. After transfection with miR-149-5p mimics or miR-149-5p mimics combined with TRADD vector into chondrocytes with or without TNF-α treatment, cell viability was measured by CCK8 assays (A); the production of inflammatory cytokines, including IL-1β, IL-6 and IL-18, was detected by ELISA assays (B–D). n = 3 in each group, *p < 0.05
Discussion
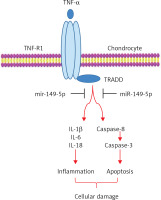
The present study attempted to investigate the underlying molecular mechanisms involved in TNF-α-induced chondrocyte apoptosis. We discovered that TNF-α stimulation resulted in the reduction of miR-149-5p expression, which had the ability to relieve the inhibitory effect of miR-149-5p on its target gene. Therefore, the activity of TRADD and its downstream target caspase-3/8 signaling were activated in TNF-α-treated chondrocytes. Next, we transfected miR-149-5p mimics into chondrocytes, and overexpression of miR-149-5p repressed TNF-α-induced growth inhibition, an increase in the secretion of IL-1β, IL-6 and IL-18 in the supernatant liquid, and the elevation of apoptotic cell proportion, which were reversed by overexpression of TRADD. These results demonstrated that TNF-α exacerbated the secretion of inflammatory cytokines, including IL-1β, IL-6 and IL-18, and apoptosis in chondrocytes could be attenuated by miR-149-5p via the inhibition of TRADD signaling (Figure 6).
Figure 6
Simplified model for how the miR-149-5p mimics inhibited TNF-α-induced apoptosis and inflammation in chondrocytes
TRADD as a transducer of TNF-α/TNFR1 signaling modulates TNF-α-induced apoptosis by recruiting RIP, TRAF2, and FADD to activate caspase-8 and caspase-3 [27]. TRADD is expressed at high levels in many different cellular models of TNF-α-induced apoptosis [11, 13, 28]. Other studies reveal that TNF/TNFR signal transduction and their downstream targets, TRADD, caspase-8 and caspase-3, are involved in injurant-induced chondrocyte apoptosis in vitro [5, 14]. In the present study, we found that TNF-α treatment dramatically suppressed cell viability and elevated the production of inflammatory cytokines and the apoptotic cell proportion in chondrocytes, and the protein expression of TRADD, TNFR1, cleaved caspase-8 and cleaved caspase-3 was up-regulated in TNF-α-stimulated chondrocyte in vitro. Therefore, TNF-α-activated TRADD and caspase-3/8 apoptosis cellular signal transduction proves tremendously intriguing for further mechanistic investigation in chondrocyte apoptosis.
In addition, it was also noted that TNF-α treatment had the ability to regulate TRADD-associated miRNA expression levels in chondrocytes. Subsequently, the mechanisms involved in the effect of TNF-α-induced apoptosis in chondrocytes were investigated. A comprehensive analysis of miRNA expression profile in TNF-α-stimulated chondrocytes indicates that 15 miRNAs and 18 miRNAs are upregulated and down-regulated, respectively [21]. In addition, TNF-α treatment leads to the down-regulation of miR-27b expression levels in human chondrocytes [29]. In our study, miR-149-5p was sensitive to TNF-α, reflecting a rapid reduction of miR-149-5p expression levels upon TNF-α stimulation, implying that miR-149-5p might be involved in TNF-α-induced chondrocyte dysfunction. In fact, miR-149-5p negatively regulated TNF-α-mediated inflammation and apoptosis in chondrocytes.
We demonstrated that TRADD was a direct target of miR-149-5p, and the anti-apoptotic effect of miR-149-5p in TNF-α-stimulated chondrocytes was neutralized by the overexpression of TRADD. Previous studies highlight that miR-149-5p plays a crucial role in mediating apoptosis-related pathways [30–32]. For example, over-expression of miR-149-5p protects against high glucose-evoked pancreatic β-cell apoptosis via repressing BH3-only Protein BIM [30]. Grieco et al. also document that miR-149-5p loss-of-function exacerbates IL-1β plus IFN-γ-induced apoptosis in human pancreatic β-cells by targeting proapoptotic BH3-only proteins DP5 and PUMA [31]. These findings suggest that miR-149-5p may function as an anti-apoptotic mediator in extrinsic pro-apoptotic stimulus-triggered cell death. However, in contrast to its anti-apoptotic role in normal cells, acute myeloid leukemia cell line THP-1 apoptosis is decreased by miR-149-5p overexpression and increased by miR-149-5p suppression; the underlying molecular mechanism was modulated by targeting the fas ligand [32]. These results indicate that miR-149-5p may perform different functions in the process of apoptosis with different diseases. In our research, up-regulation of miR-149-5p attenuated TNF-α-evoked chondrocyte apoptosis through post-transcriptional repression of TRADD. These results suggest that the miR-149-5p/TRADD pathway might be a novel therapeutic strategy for the treatment of OA.
In conclusion, we elucidated a novel regulatory mechanism in which miR-149-5p as a post-transcriptional regulator inversely regulated TNF-α-mediated chondrocyte growth inhibition, apoptosis and the inflammatory response by inhibiting TRADD cascade signaling.