Introduction
Uterine fibroids (UFs) are benign tumors which are derived from the smooth muscle cells of the uterus or the vascular wall. The UFs are one of the most common pathologies in women globally. Their incidence has been estimated at approximately 25–80%, depending on various risk factors [1–4]. The UFs affect over 25% of females in the reproductive age and cause significant morbidity in that population. The UFs can be the cause of many clinical symptoms including abnormal uterine bleeding, pelvic pressure-related complaints, pain, urinary incontinence, urine retention, infertility, and others [3, 5, 6].
Vitamin D comprises a group of fat-soluble steroid compounds which exert powerful, pleiotropic effects all over the human body [7, 8]. Vitamin D receptors are found in many different tissues, e.g. brain, prostate and immune system organs. Vitamin D is believed to reduce the risk of several chronic diseases, chief among them diabetes, cardiovascular diseases and multiple sclerosis. Vitamin D has been postulated to reduce the incidence of breast, prostate and colorectal cancers, probably due to better regulation of cell differentiation, angiogenesis inhibition and apoptosis stimulation [7–11].
Recent studies have demonstrated that UF development can be particularly related to vitamin D [4, 12–15]. According to the available sources, patients with UFs have statistically significantly lower 25-hydroxyvitamin D (25(OH)D) serum concentrations in comparison to controls [4, 14, 16]. Thus, vitamin D may be a potential protective factor against the development of UFs [4, 17].
The pleiotropic actions of vitamin D are mediated by a specific type of receptor [15, 18]. The VDR belongs to the family of nuclear receptors and carries out its functions as a transcription factor. Over 900 genes may be transcribed by VDR [19–21]. The active form of vitamin D – 1,25-dihydroxyvitamin D – mediates its biological functions through a steroid transcriptional mechanism by influencing the expression of several associated genes. In its metabolic pathway, VDR undergoes a conformational change and forms a heterodimer complex with the retinoid X receptor (RXR). The RXR belongs to the family of nuclear receptors specific for steroid hormones (SHs), which also includes thyroid hormone receptors and peroxisome proliferator-activated receptor – PPAR. The heterodimer with RXR binds to specific vitamin D promoter-dependent gene sites, called vitamin D response elements (VDREs), and then influences transcriptional activity [19–22].
Human VDR is encoded by a VDR gene located on chromosome 12 (12cen-q12). Not all functional effects of described VDR polymorphisms have been revealed [19, 20, 22, 23]. Published data showed that the length of the protein product of the VDR gene could influence the transcriptional rate of vitamin-dependent genes [24, 25]. Recent studies have focused on the possible role of VDR genetic variations in the development of several types of diseases, e.g. autoimmune system diseases, various cancers and infections [20, 26–28]. Furthermore, some of the VDR gene SNP (single nucleotide polymorphism) polymorphisms are associated with a different response to therapies in humans, e.g. in osteoporosis [29]. Three SNPs, rs731236 (TaqI), rs1544410 (BsmI) and rs2228570 (FokI), were proven to be important in the pathophysiology of selected diseases of the female reproductive tract [15, 30, 31]. Both rs731236 and rs1544410 were found to be useful predictors of genetic susceptibility for earlier surgical menopause [32]. According to Shahbazi and Gulec Yilmaz et al., certain haplotypes of rs2228570 are associated with an elevated risk for UFs in Iranian and Turkish populations, respectively [15, 33].
It is commonly known that UFs are associated with familial occurrence [3, 4, 34]. In addition to the current data about the risk for UFs associated with mutations within the MED12 gene, there are also some data indicating that some genetic variants may affect family history [35, 36]. In light of the very promising reports of an unquestionable relationship between vitamin D pathways and UF development, our team decided to investigate the relationships between the abovementioned SNPs and an elevated UF risk for specific haplotypes.
The aim of this study was to evaluate the association between the rs731236 (TaqI), rs1544410 (BsmI), and rs2228570 (FokI) SNPs and the incidence of UFs in Polish Caucasian women. Frequencies of selected VDR polymorphisms and their association with UFs in the homogeneous Polish population are presented in this manuscript.
Material and methods
Subjects
The study was conducted at our gynecological department. The Local Ethics Committee approved the study. A total of 197 Caucasian women of Polish origin were included in the study. Patients admitted to the hospital for UF-related surgery comprised the study group while controls were recruited from patients of the hospital outpatient clinic without UFs. The subjects formed an ethnically homogeneous population. All participants signed an informed consent form and completed a short questionnaire regarding sociodemographic, medical (including chronic illnesses) and gynecological data (including use of combined oral contraception in the past).
A subject was deemed eligible for the study group if she presented with at least one UF on an ultrasound scan. All gynecological ultrasound examinations were conducted by an experienced ultrasonographer (transvaginally and/or transabdominally) in two-dimensional gray-scale presentation. A UF was defined as a hypoechogenic and heterogeneous symmetric area with distinct, visible margins. Patients were excluded if UFs could not be visualized or if adenomyosis was suspected. The control group comprised subjects free of uterine pathology, confirmed by ultrasound scan (especially UFs). Among the recruited patients, 114 were fibroid-positive and 83 were fibroid-negative. Patients with a history of malignancy or an active malignancy were excluded from the study.
Genotyping
Genomic DNA was extracted from peripheral blood leukocytes in all subjects using the Extractme DNA blood kit (Blirt S.A. Gdansk, Poland), according to the manufacturer’s instructions. DNA concentration and purity were determined with a Quawell Q5000 micro-volume UV-Vis spectrophotometer, with the measuring absorbance ratio of 260 nm/280 nm. The rs731236 (TaqI) and rs2228570 (FokI) polymorphisms were determined using TaqMan probes. These assays, designed to detect the rs2228570 (C__12060045_20) and rs731236 (C__2404008_10) SNPs, were purchased from Life Technologies and applied using the ViiA 7 Real-Time PCR System (Life Technologies, USA). Real-time PCR experiments were performed according to the manufacturer’s instructions, with the final reaction volume of 15 μl which contained 20 ng of genomic DNA, 7.5 μl of TaqMan Genotyping Master Mix (2×) (Applied Biosystems, USA), and 0.75 μl of assay mix (20×). Real-time PCR thermal conditions were as follows: initial denaturation at 95°C for 10 s and 40 cycles of 95°C for 15 s (denaturation) and 60°C for 60 s (annealing/extension).
The VDR rs1544410 (BsmI) polymorphism was determined using LightSNiP assay designed by TIB-MolBiol (Berlin, Germany). Real-time PCR was performed with the LightCycler 480 instrument available from Roche Applied Science, with the final reaction volume of 15 μl, which contained 20 ng of genomic DNA, 1 μl of LightSNiP reagent mix, 10 μl of LightCycler 480 Probes Master Mix, and 4 μl of PCR grade water. Real-time PCR thermal conditions were as follows: 10 min for initial denaturation at 95°C, 45 cycles at 95°C for 10 s for further denaturation, 15 s at 60°C for annealing, and 15 s at 72°C for extension. Melting curve analysis was performed with the initial denaturation at 95°C for 30 s, followed by melting from 40°C to 75°C with the ramp rate of 0.19 °C/s and a final cooling at 40°C for 30 s. The results were analyzed using the ViiA 7 Software (Applied Biosystems, USA) and LightCycler 480 Software (Roche). The samples were run in duplicate, with positive, negative controls and blanks. Over 50% of the samples were determined twice, and genotyping was 100% concordant.
Statistical analysis
All statistical calculations were performed with the Statistica software (version 12.0). The distribution of variables was tested with the Shapiro-Wilk test. Differences in continuous parameters among the subgroups were tested using the Mann-Whitney U-test. Non-continuous variables were tested with the χ2 test. Allele frequencies for VDR variants were calculated with the gene counting method. Hardy-Weinberg equilibriums were tested to compare the observed and the expected genotype and allele frequencies. Subjects with BMI ≥ 30 kg/m2 were considered as obese according to World Health Organization criteria [37]. Logistic regression analysis was used to find the determinants for UF occurrence risk: family history of fibroids, obesity, hypertension, diabetes mellitus type 2, hypothyroidism, asthma and combined oral contraceptives (COC) use in the past. The p-value of < 0.05 was assumed as statistically significant.
Results
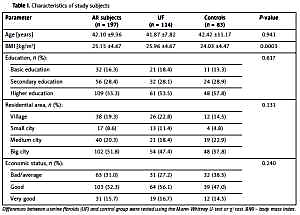
The study included a total of 197 Polish Caucasian women, aged from 22 to 70 (mean age: 42.10 ±9.36 years) and BMI range 17.7–39.06 kg/m2. Demographic characteristics of the subjects are presented in Table I. The Mann-Whitney U-test showed statistically significant differences in mean weight and body mass index (BMI) values between the groups (p = 0.0005 and p = 0.0003, respectively). No statistically significant differences in age and height between UF patients and healthy controls were found.
Table I
Characteristics of study subjects
Among all subjects, the allele distribution of the VDR gene polymorphisms was in the Hardy-Weinberg equilibrium (p > 0.05). The χ2 test was performed to assess differences in genotype frequency between UF patients and healthy controls. The frequency of rs1544410 GG genotype (wild) was 45.6% in UF subjects and 39.8% in controls. Higher occurrence of rs731236 TT (wild) genotype was observed among UF subjects than in controls, 47.4% vs. 41%, respectively. However, no significant differences in genotype and allele frequencies of the studied VDR gene polymorphisms were found. Distribution of genotype frequencies is presented in Table II.
Table II
Distribution of genotype frequencies of selected VDR gene polymorphisms in uterine fibroid positive patients and control group
Logistic regression analysis identified family history of UFs and obesity as two major risk factors for UF. Results of the logistic regression model adjusted for age are presented in Table III. There was no association of UF occurrence with chronic illnesses reported most often in the studied population (hypertension (near statistical significance), diabetes mellitus type 2, hypothyroidism, asthma) and COC use in the past. A family history of UFs and obesity remained significant predictor factors of UFs in studied subjects. The odds of UFs in women with a family history of UFs were 3.5 times greater than the odds of UFs in women without family history of UFs. The odds of UFs in women with obesity were 3.85 times higher than those in non-obese subjects.
Table III
Odds ratio for the presence of UFs among studied subjects
Discussion
Vitamin D receptors can be found all over the human body. VDRs have been identified in both tissues and organs, and not only in those associated with calcium homeostasis and bone metabolism [7, 8, 38]. According to numerous reports, it seems that vitamin D may be involved in many biological processes other than mineral homeostasis [8, 38, 39]. The VDR polymorphisms have been associated with autoimmune diseases such as diabetes mellitus, inflammatory bowel diseases and pulmonary diseases [40, 41].
Wise et al. investigated UF incidence in relation to polymorphism in genes involved in vitamin D metabolism and skin pigmentation in selected women [42]. To the best of our knowledge, it was the first study to evaluate the relationship between genes involved in vitamin D metabolism and UF incidence. These authors evaluated the risk for fibroids in Afro-American women. There were 12 SNPs in 8 genes, also in VDR – rs739837 and rs886441. All of these genes are involved in important metabolic pathways in humans [42]. Wise et al. identified two SNPs – rs12800438 near DHCR7 and rs6058017 in ASIP – which are significantly associated with UF occurrence [42]. It is believed that both abovementioned polymorphisms are associated with vitamin D deficiency [43, 44]. Didriksen et al. suggest that genetic polymorphisms in vitamin D-related enzymes may greatly influence the serum levels of 25(OH)D [45], and hypovitaminosis D is a confirmed risk factor for the occurrence and growth of UFs [4, 14, 16, 17].
In a study by Halder et al., UFs express reduced levels of VDRs in comparison to the normal myometrium [46]. An inverse correlation between VDR levels and up-regulated estrogen and progesterone receptors has been observed in UFs, suggesting that vitamin D functions as an antagonist of sex SH receptors in UF tumor tissue [47]. Vitamin D takes part in the regulation of SH synthesis by interacting with its nuclear receptor [48]. A large number of the therapeutic effects of vitamin D are the result of its negative gene regulation. VDR ligands have been documented to inhibit the expression of cytokines, e.g. interleukins, tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β), or interferon [49]. TNF-α is an important cytokine associated with patient symptoms and complaints [50]. Abnormal concentrations and overexpression of TGF-β are responsible for some clinical symptoms associated with UFs [51]. Possibly, reduced vitamin D serum levels result in the loss of vitamin D functions, which in turn disturbs VDR action and decreases its expression [4, 12, 15].
During the literature search, the rs731236, rs1544410 and rs2228570 SNPs attracted our attention, as they may be relevant with regard to VDR and SH-related female diseases. The rs731236 polymorphism is a synonymous site, a single base change of thymine (T) to cytosine (C) in codon 352 at the 3′ end of the VDR gene [52]. In the study by Barroso et al., the synonymous variant of rs731236 appeared to be associated with protection from breast cancer [53]. However, the study by Gapska et al. failed to support this finding in the population of Polish women [54]. A more recent study by Siddamalla et al. found an association between the above-mentioned SNPs and the risk of inheritable polycystic ovary syndrome (PCOS) [31].
The rs2228570 polymorphism is located in exon 2 in the 5′ coding region of the VDR gene. That polymorphism acts by changing the translation initiation site, due to T to C substitution. This change generates long and short variants of VDR [55]. Lurie et al. presented data indicating that rs2228570 polymorphism might influence ovarian cancer susceptibility [56]. A study by Tworoger et al. further supported the role of the vitamin D pathway, including VDR, in ovarian carcinogenesis [30]. On the other hand, Anderson et al. found an association between rs2228570 and reduced risk of breast cancer [57]. Finally, in his recent publication, Shahbazi found that rs2228570 was associated with UF development in Iranian women [15], which is consistent with the recent analysis performed on Turkish women [33]. Moreover, the importance of rs2228570 polymorphism has been shown in Saudi breast cancer patients [58]. Carriers of the A allele are more likely to develop breast cancer. These authors concluded that the vitamin D pathway is a potentially important mediator of breast cancer risk and this relationship seems to be modified by estrogen receptor status of the tumor [58].
The SNP rs1544410 includes an intronic replacement which results in a synonymous change G (guanine) to A (adenine) at codon 352 [59]. Some of the rs1544410 haplotypes may constitute an inheritable risk of PCOS in South Indian women (as in the case of rs731236) [31]. In 2006, Dvornyk et al. presented evidence that homozygotes at the minor alleles of rs1544410 and rs731236 had higher risk of early surgical menopause [32]. These authors concluded that some of the vitamin D pathways might have a potential effect on the occurrence of diseases which results in surgical menopause. UFs were one of the most important indications to perform the hysterectomy in this study [32]. It remains unknown how VDR expressed in the reproductive organs influences their functions, but according to the available data, it alters the onset of menopause.
In our study, we did not find any statistically significant differences between UF-positive women and controls in the case of rs731236, rs1544410 and rs2228570 VDR gene variants. This demonstrates that UFs are present irrespectively of VDR polymorphism status in Polish Caucasians. As demonstrated in a different study by our group, 25(OH)D serum levels are lower in Polish Caucasian patients who are fibroid-positive [4]. It can be supposed that vitamin D receptor polymorphism (rs731236, rs1544410, and rs2228570) may not be an important predictor of the 25(OH)D serum levels in UF subjects.
As expected, in our study, obesity was associated with an increased risk of UFs (OR = 3.85). It is consistent with the previous reports [60]. Additionally, a family history of UFs was associated with an increased risk of UFs (OR = 3.50). In the studied population we did not observe any relationship between hypertension, diabetes mellitus type 2, asthma, hypothyroidism or COC use in the past and risk of UF occurrence.
Despite the positive results regarding the increased risk for UFs in the selected VDR polymorphisms obtained by Shahbazi [15] and Gulec Yilmaz et al. [33], our larger sample size study did not confirm these findings. The difference may have resulted from the ethnicity of the investigated populations. Still, it remains unclear whether ethnicity or polymorphism of other genes has an impact on UF incidence. Thus, more research is vital to elucidate the matter further. In light of the obtained results, it is safe to conclude that rs731236, rs1544410 and rs2228570 polymorphisms do not affect the risk of UFs in women. However, due to the diversity of biochemical and genetic pathways in the pathophysiology of UF, polymorphisms of a single gene cannot be held responsible and the search should be continued. The ultimate consensus about the role of the VDR variants requires multicenter studies with a larger sample size in order to generate more reliable results.
Our study does have some limitations. We did not obtain data on the lifestyle (e.g. physical activity, sunbathing habits) and dietary habits, which may have an impact on UF occurrence [61, 62].
In conclusion, our study revealed no significant associations between rs731236, rs1544410 and rs2228570 VDR polymorphisms and UF incidence in a homogeneous group of Polish Caucasian women. Larger sample size and multi-ethnic studies are necessary to fully understand the role of VDR in UF metabolism and growth.