Introduction
Atherosclerosis, the leading cause of acute myocardial infarction and stroke, engenders enormous global health and economic burdens. It is well established that atherosclerosis is a chronic inflammatory disease of the vascular wall [1]. Monocytes and macrophages have key functions in every phase of the atherosclerosis process, from the formation of the fatty streak to the destabilization and rupture of the plaque [2]. The recruitment and migration of monocytes to the vascular wall represents the hallmark of atherogenesis and requires complex interactions between cell adhesion molecules and chemotactic factors [2].
Urotensin II (UII), initially isolated from goby urophysis, has been regarded as one of the most potent vasoconstrictor peptides [3]. UII exerts various behavioral effects through binding to G-protein-coupled receptor 14, termed UT [3]. Increased plasma levels of UII and elevated expression of UII and UT have been demonstrated in several cardiovascular diseases, including essential hypertension, atherosclerosis, congestive heart failure and pulmonary hypertension [3]. In addition, UII and UT are predominantly expressed in atherosclerotic plaques of the aorta, the coronary arteries and the carotid arteries [3]. Clinical observations have revealed that UII levels are correlated with carotid plaque formation risk in patients with essential hypertension and type 2 diabetes [4, 5]. In vitro, in addition to promoting endothelial cells (EC) and smooth muscle cell (SMC) proliferation [6, 7], UII exerts a pro-inflammatory effect on vascular wall cells. Urotensin II has been reported to have a chemoattractant effect on monocytes and can accelerate macrophage-derived foam cell formation [8, 9].
Our previous work revealed that UII upregulates the expression of 5-lipoxygenase (5-LO) in rat vascular adventitial fibroblasts [10, 11]. 5-LO is the key enzyme for the biosynthesis of leukotriene B4 (LTB4). LTB4 is generated from arachidonic acid and is a potent chemoattractant that facilitates leukocyte adhesion and recruitment to endothelial cells, which plays a well-established role in the pathogenesis of atherosclerosis [11]. However, it is unclear whether UII regulates 5-LO expression and LTB4 production in macrophages. In the present study, we explored the role of UII in regulating the 5-LO/LTB4 axis in macrophages.
Material and methods
Materials
The reagents used in the study were purchased from the following suppliers: Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS) and trypsin (Gibco); mouse UII (Phoenix Pharmaceuticals); urantide (Peptides International); Invitrogen TRIZOL reagent (Life Technologies); High-Capacity cDNA Reverse Transcription Kit and SYBR Select Master Mix (Applied Biosystems); mouse LTB4 ELISA kit (Cayman); antibodies against 5-LO, p-Akt, Akt (CST), and β-actin (Beijing TransGen Biotechnology); LY294002 (CST); 2′,7′-dichlorofluorescein diacetate (DCFH-DA); N-acetylcysteine (NAC) and diphenyliodonium (DPI) (Sigma); goat anti-rabbit secondary antibody and rabbit anti-mouse second antibody (Beijing Zhongshan Golden Bridge Biotechnology), and SuperEnhanced chemiluminescence detection reagents (Millipore).
Cell culture
The RAW264.7 cell line was purchased from the Cell Center of Peking Union Medical College. The cells were cultured in DMEM containing 10% FBS, 100 mg/ml streptomycin and 100 units/ml penicillin at 37°C in a humidified atmosphere with 5% CO2/95% air. After reaching confluence, the cells were passaged at a 1 : 4 to 1 : 6 ratio. All experiments were conducted using cells in the logarithmic growth phase.
Enzyme-linked immunosorbent assay
The ELISA was performed with a mouse LTB4 ELISA Kit to assess the release of LTB4 into the culture medium. Briefly, after treatment with the respective stimuli, 200 µl of culture medium was collected and then centrifuged to obtain the supernatant. The ELISA was conducted according to the manufacturer’s directions. The absorbance was read at 450 nm.
Real-time PCR
Total RNA was extracted from cultured RAW264.7 cells following the manufacturer’s directions and then reverse-transcribed into cDNA as described. Real-time PCR was performed using the ABI 7300 System (Applied Biosystems). The expression levels of β-actin and 5-LO were assessed using the following primer sequences: β-actin, F 5′-GGCCAACCGTGAAAAGATGA-3′ and R 5′-CACAGCCTGGATGGCTACGT-3′ and 5-LO, F 5′-CTGCTGTGCATCCCCTTTTC-3′ and R 5’-CTGTTCCCGGGCCTTAGTGT-3′. The relative mRNA levels of 5-LO in RAW264.7 cells were determined using the comparative threshold cycle (CT) method using the 2–ΔΔCT equation. β-actin was used as an internal control gene. Three different experiments were performed for each experimental condition.
Western blotting
Cell lysates containing 30 µg of total protein were resolved by electrophoresis in a 10% sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel and transferred to a nitrocellulose membrane. After blocking with 5% nonfat milk, the membranes were probed with specific primary antibodies targeting 5-LO (1 : 1000 dilution), β-actin (1 : 5000 dilution), p-Akt (1 : 1000 dilution), or Akt (1 : 1000 dilution) overnight at 4°C. After rinsing, the membranes were detected with goat anti-rabbit secondary antibody (1 : 10000 dilution) or rabbit anti-mouse secondary antibody (1 : 10000 dilution) followed by enhanced chemiluminescence.
Measurement of ROS
Intracellular reactive oxygen species (ROS) production by RAW264.7 macrophages was determined using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). The cells were pretreated with different stimuli for 1 h prior to exposure to UII for 2 h. Next, the cells were loaded with DCFH-DA (10 µM) and incubated at 37°C for 30 min, followed by triple washes with FBS-free medium. The fluorescence intensity was immediately read using a fluorescence plate reader with an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The ROS levels were calculated as the relative increase compared with the control.
Statistical analysis
Values are expressed as the mean ± SEM. One-way analysis of variance was applied to determine the differences between groups. Student’s t test was used for multiple comparisons. The data were analyzed using SPSS Statistics 16.0 software (SPSS Inc. Chicago, USA). A p-value less than 0.05 was considered statistically significant.
Results
UII promotes LTB4 release in macrophages
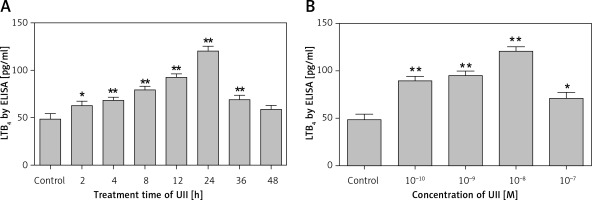
To investigate whether UII could regulate LTB4 production in RAW264.7 macrophages, ELISA assays were performed. The results showed that UII was able to promote LTB4 production in a time- and concentration-dependent manner in RAW264.7 macrophages. As shown in Figure 1 A, when the cells were treated with 10–8 M of UII, LTB4 production was increased after 2 h of treatment, peaking at 24 h of treatment. As shown in Figure 1 B, LTB4 production was significantly increased after stimulation with different concentrations of UII for 24 h. The maximal response was reached at 10–8 M (Figure 1 B).
Figure 1
UII promotes LTB4 release into the supernatant of RAW264.7 cells. A – Effect of UII (10–8 M) on LTB4 production after the indicated incubation times. The cells were left untreated (control) or treated with UII for different durations. B – Effect of 24 h treatments with different concentrations of UII on LTB4 production. The cells were left untreated (control) or treated with UII for 24 h at the indicated concentrations
Values are the means of the LTB4 protein concentration in pg/ml ± SD. The data are from three independent experiments and are expressed as the mean ± SEM. *P < 0.05 vs. control; **p < 0.01 vs. control.
UII promotes 5-LO expression in macrophages
Because 5-LO is the rate-limiting enzyme in LTB4 generation, we also explored whether 5-LO is regulated by UII. We found that the expression of 5-LO was upregulated by UII in both a concentration- and a time-dependent manner. Figures 2 A and C show that 10-8 M UII promoted 5-LO mRNA and protein expression in a time-dependent manner, with peak levels reached after 24 h of treatment. Figures 2 B and D show that different concentrations of UII upregulated 5-LO mRNA and protein expression in cells, with the maximal response reached at a concentration of 10–8 M. The changes in 5-LO protein expression in response to UII correlated well with the UII-induced LTB4 production shown in Figures 1 A and B, indicating that the UII-induced LTB4 production may be 5-LO dependent. Thus, unless otherwise illustrated, UII was applied at 10–8 M for 24 h in the following experiments.
Figure 2
UII promotes 5-lipoxygenase (5-LO) expression in RAW264.7 macrophages. A – Time course of UII-stimulated 5-LO expression determined by real-time PCR. The cells were incubated with 10–8 M UII for the indicated times. B – Concentration-response relationship of UII with 5-LO expression in RAW264.7 macrophages via UT determined by real-time PCR. The cells were incubated with different concentrations of UII for 24 h or pretreated with urantide (10 μM) for 1 h prior to stimulation with UII (10–8 M) for 24 h. C – Time course of UII-stimulated 5-LO expression determined by western blot analysis. D – Concentration-response relationship of UII with 5-LO expression in RAW264.7 macrophages via UT determined by western blot analysis
UA – urantide. The data are from three independent experiments and are expressed as the mean ± SEM. *P < 0.05 compared with control; **p < 0.01 compared with control; #p < 0.01 compared with the UII (10–8 M) group.
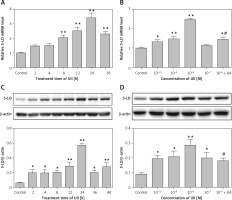
We next found that blocking UT with urantide (10 µM) significantly reduced the UII-induced 5-LO expression. These results indicate that UII promotes 5-LO expression in RAW264.7 macrophages via UT (Figures 2 B, D).
Involvement of ROS in UII-induced 5-LO expression and LTB4 release in RAW264.7 macrophages
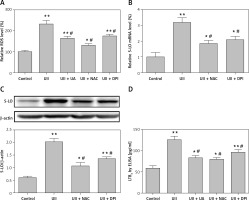
Reactive oxygen species play important roles as signaling molecules in the pathophysiology of complex cardiovascular diseases [12]. UII has been demonstrated to promote NADPH oxidase-derived ROS generation [13]. Moreover, NADPH oxidase-derived ROS can regulate 5-LO expression and LTB4 synthesis in murine alveolar macrophages [14]. To determine whether intracellular ROS participated in the UII-induced 5-LO expression, intracellular ROS generation was assessed. As shown in Figure 3 A, exposure of RAW264.7 macrophages to UII for 2 h resulted in a noticeable increase in dichlorofluorescin (DCF) fluorescence compared with the control, whereas pretreatment with urantide (10 µM), the antioxidant NAC (10 mM) and the NADPH oxidase inhibitor DPI (10 µM) for 1 h clearly attenuated this effect. Further investigation revealed that elimination of ROS by NAC and DPI significantly inhibited UII-induced 5-LO expression at the gene and protein levels (Figures 3 B, C) as well as LTB4 release (Figure 3 D). These results revealed that NADPH-derived ROS may be involved in UII-induced 5-LO expression in RAW264.7 macrophages.
Figure 3
Involvement of ROS in UII-induced 5-LO expression and LTB4 release in RAW264.7 macrophages. A – Effect of the different inhibitors on the UII-stimulated generation of ROS. The cells were stimulated with UII (10–8 M) for 2 h after pretreatment with urantide (10 μM), NAC (10 mM) and DPI (10 μM) for 1 h and then incubated for 30 min with DCFH-DA (10 μM). B – Effect of NAC and DPI on UII-induced 5-LO mRNA expression determined by real-time PCR. The cells were stimulated with UII (10–8 M) for 24 h after pretreatment with NAC (10 mM) and DPI (10 μM) for 1 h. C – Effect of NAC and DPI on UII-induced 5-LO protein expression determined by western blot analysis. D – Effect of different inhibitors on LTB4 release in the presence of UII (10–8 M) for 24 h
UA – urantide. The data are from three independent experiments and are expressed as the mean ± SEM. *P < 0.05 compared with control; **p < 0.01 compared with control; #P < 0.01 compared with the UII (10–8 M) group.
Akt mediates UII-induced expression of 5-LO and LTB4 release in RAW264.7 macrophages
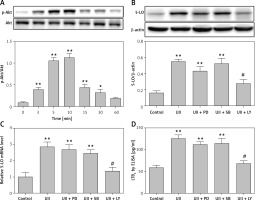
UII regulates the production of inflammatory mediators by sequentially promoting ROS generation and activation of the Akt pathway [13]. 5-LO expression can be induced in monocyte cells by inflammatory stimuli via an Akt-dependent pathway [15]. To investigate whether the Akt pathway was involved in UII-induced 5-LO expression and LTB4 release, we determined the phosphorylation status of Akt in RAW264.7 macrophages after UII treatment. As shown in Figure 4 A, phosphorylated Akt increased markedly following stimulation of the cells with UII. In contrast, pretreatment with the Akt inhibitor LY294002 (10 µM) for 1 h before UII incubation antagonized the UII-induced 5-LO expression (Figures 4 B and C) and LTB4 production (Figure 4 D) in RAW264.7 macrophages. However, PD98059 (an ERK inhibitor) and SB203580 (a p38MAPK inhibitor) failed to rescue UII-induced 5-LO synthesis (Figures 4 B and C) and LTB4 production (Figure 4 D). These results together indicate that activation of the Akt signaling pathway is required in UII-induced 5-LO/LTB4 inflammation.
Figure 4
Akt mediates UII-induced expression of 5-LO and LTB4 release in RAW264.7 macrophages. A – UII induces Akt phosphorylation in RAW264.7 macrophages at the indicated time points. The cells were exposed to 10–8 M UII for 0 to 60 min. B, C – Effect of the different inhibitors on UII-induced 5-LO expression. The cells were pretreated with PD98059 (10 μM), SB203580 (10 μM) and LY294002 (10 μM) for 1 h prior to stimulation with UII (10–8 M) for 24 h. 5-LO mRNA expression (C) and protein level (B) were then detected by real-time PCR and western blot analysis, respectively. D – Effect of different inhibitors on LTB4 release in the presence of UII (10–8 M) for 24 h
UA – urantide, PD – PD98059, SB – SB203580, LY – LY294002. The data are from three independent experiments and are expressed as the mean ± SEM. *P < 0.01 compared with control; #p < 0.01 compared with the UII (10–8 M) group.
UII induces LTB4 release in macrophages via the UT/ROS/Akt signaling cascade
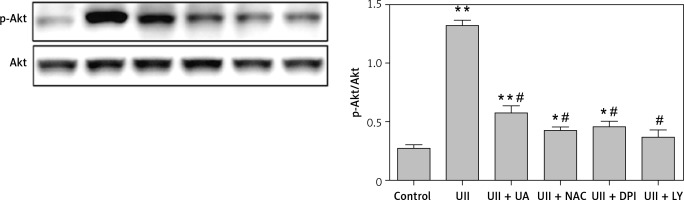
We next sought to investigate whether the UT/ROS pathway is associated with UII-induced Akt activation. We pretreated RAW264.7 cells with urantide (10 µM), NAC (10 mM), DPI (10 µM) or LY294002 (10 µM) for 1 h prior to exposing the cells to UII for 10 min. We observed that blocking UT or scavenging ROS markedly inhibited UII-induced Akt phosphorylation (Figure 5), indicating that the UII-induced activation of Akt is UT/ROS dependent.
Figure 5
Effects of different inhibitors on the phosphorylation level of Akt. The cells were stimulated with UII (10–8 M) for 10 min after pretreatment with urantide (10 μM), NAC (10 mM), DPI (10 μM), and LY294002 (10 μM) for 1 h. Total and phosphorylated Akt levels were determined by western blot analysis
UA – urantide, LY – LY294002. The data are from three independent experiments and are expressed as the mean ± SEM. *P < 0.01 compared with control; #P < 0.01 compared with the UII (10–8 M) group.
Taken together, our results reveal that UII is a pro-inflammatory factor that promotes 5-LO-dependent LTB4 production. This effect is mediated through a novel UT/ROS/Akt pathway and may play a crucial role in adventitial inflammation.
Discussion
Monocytes/macrophages play crucial and direct roles in all atherosclerosis lesional stages. UII has chemoattractant properties for monocytes, and LTB4 has chemotactic activity for macrophages. However, the relationship between UII and LTB4 in macrophages is unclear. In the present study, we investigated whether UII could promote LTB4 release from a macrophage-like cell line, with a special focus on the relative mechanisms. We reveal for the first time that the pro-inflammatory factor UII induces 5-LO-dependent LTB4 production through the UT/ROS/Akt pathway in macrophages. The study suggests that UII may play a key role in macrophage activation.
LTB4 is an arachidonic acid metabolite that is produced via the 5-lipoxygenase pathway. It is also a pro-inflammatory mediator that is involved in the pathogenesis of certain inflammatory diseases [16]. 5-LO is the rate-limiting enzyme in LTB4 synthesis. 5-LO has been linked to the pathophysiology of atherosclerosis [17], and a 5-LO promoter polymorphism has been correlated with atherosclerosis [18]. 5-LO inhibition can reduce macrophage recruitment to atherosclerotic lesions and may contribute to the amelioration of lesion formation [19]. In the present study, we found that UII promoted LTB4 secretion and 5-LO expression in a time- and concentration-dependent fashion. UII at 10–8 M showed the maximal response at 24 h after the initiation of treatment but there was no effect on 5-LO mRNA expression when UII was applied at the concentration 10–7 M. We further found that UII at 10–7 M promoted 5-LO mRNA expression in a time-dependent manner, with the peak effect observed after 8 h of treatment; the response then decreased to the baseline level at 24 h (data not shown). At the protein level, however, 5-LO and LTB4 continued to increase after 24 h of 10–7 M UII stimulation. The discrepancy between 5-LO mRNA and protein levels after 10–7 M UII stimulation for 24 h suggests that the pathways and mechanisms by which 5-LO mRNA and protein are upregulated may be different. This regulatory mode is similar to the 4-hydroxynonenal (HNE)-induced expression of 5-LO [20], in which 5-LO mRNA expression peaked after 4 h of treatment and decreased gradually, but 5-LO protein was still increasing after 24 h of treatment. As 5-LO is the limiting enzyme for both LTB4 and LTC4 and UII promoted the expression of 5-LO and LTC4 release in fibroblasts in our previous study [10], we predict that UII may also enhance LTC4 production in macrophages and UII may be involved in various biological effects on macrophages. Further studies need to be performed. The UT blocker urantide partially reduced the UII-induced increase in LTB4 production and 5-LO expression. These results demonstrate that UII exerts its effects via UT and indicate the need for a more specific and potent UT blocker. This finding may explain the transmigration of macrophages to inflammatory sites during the pathogenesis of atherosclerosis. Additionally, these findings enrich our knowledge regarding the pro-inflammatory effects of UII. A large number of 5-LO-expressing macrophages have been detected in the adventitia in lesions of apolipoprotein E (Apoe–/–) mice [21], indicating a possible role of UII in adventitial inflammation.
Previously, ROS have been regarded as a destructive factor that contributes to cardiovascular disease. However, accumulating evidence shows that ROS signaling plays an important role in a large number of cardiovascular diseases [12]. UII increases ROS generation and regulates inflammatory mediator production through multiple signaling cascades, including the Akt pathway [13]. The results of the present study indicate that UII-induced ROS generation in RAW264.7 macrophages is involved in a UT and NADPH oxidase-derived ROS pathway. These data are also consistent with the findings reported by Djordjevic et al. for pulmonary artery smooth muscle cells (PASMCs) [13]. Moreover, we found that UII stimulates 5-LO expression in macrophages via UT-mediated NADPH oxidase-derived ROS production. Coffey et al. also reported that 5-LO expression and LTB4 synthesis can be regulated in an NADPH oxidase-derived ROS-dependent manner in murine alveolar macrophages [14]. According to these data, antioxidant drugs may represent a new therapeutic target for the treatment of related inflammatory diseases.
5-LO expression is regulated in a complex manner that involves different signaling pathways. In particular, inflammatory stimuli induce 5-LO expression in monocyte cells through an Akt-dependent pathway [15]. Additionally, UII has been shown to activate the Akt signaling pathway [13, 22]. In the present study, UII-induced LTB4 release and 5-LO expression in RAW264.7 macrophages were dependent on Akt signaling but not MAPK signaling. This finding was not entirely consistent with previous results obtained using PASMCs or rat aortic adventitial fibroblasts, which indicated that UII-induced plasminogen activator inhibitor-1 (PAI-1) expression is mediated by the activation of MAPKs (mitogen-activated protein kinase) and Akt [13] and that UII regulates 5-LO expression through p38MAPK (p38 mitogen-activated protein kinase) and ERK (extracellular signaling regulatory protein kinase) pathways [10], respectively. This discrepancy suggests that the expression and regulation of 5-LO is cell type-specific and pathway-specific. We also found that UII induced the production of ROS and blockage of this production by NAC and DPI partially decreased the UII-induced phosphorylation of Akt in macrophages, suggesting that ROS affects the Akt signaling and 5-LO expression during the process.
In conclusion, our data demonstrate the ability of UII to promote LTB4 production in macrophages. This effect is most likely mediated by the UT-ROS-Akt signaling pathway. These results contribute to our understanding of the pro-inflammatory effects of UII and may provide new insights regarding the mechanism underlying the inflammatory processes of atherosclerosis.


