Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL PHARMACOLOGY / CLINICAL RESEARCH
Uncovering the role of RPL8 in glutathione synthesis-dependent ferroptosis control in hepatocellular carcinoma
1
Department of Oncology, Minhang Branch, Zhongshan Hospital, Fudan University, Shanghai, China
2
Department of Ultrasound, Eastern Hepatobiliary Surgery Hospital, The Third Affiliated Hospital of the Naval Medical University, Shanghai, China
These authors had equal contribution to this work
Submission date: 2024-04-12
Final revision date: 2024-07-19
Acceptance date: 2024-07-30
Online publication date: 2024-08-05
KEYWORDS
TOPICS
ABSTRACT
Introduction:
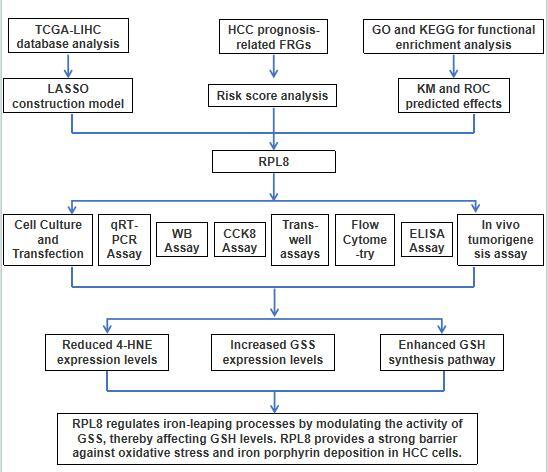
Hepatocellular carcinoma (HCC) is significantly impacted by ferroptosis, a form of regulated cell death linked to iron metabolism and oxidative stress. This process is modulated by glutathione (GSH) levels. This study explored the role of ferroptosis-related genes (FRGs) in HCC and evaluated the impact of RPL8 on HCC progression and ferroptosis mechanisms.
Material and methods:
Through comprehensive TCGA-LIHC data analysis, we identified significant FRGs affecting HCC prognosis. The expression and functional significance of RPL8 in HCC were further evaluated using cell line models and tumor xenograft experiments, focusing on its effects on cell proliferation, invasion, migration, apoptosis, and regulation of ferroptosis.
Results:
Elevated RPL8 expression was observed in HCC tissues and cell lines. Functional experimental results showed that RPL8 regulation significantly affects cell proliferation, invasion, migration, and apoptosis. RPL8 silencing significantly inhibited tumor growth in tumor xenograft experiments. Furthermore, RPL8 knockdown resulted in increased 4-HNE levels, indicating increased lipid peroxidation and triggering ferroptosis, which was partially alleviated by inhibitors such as Nec-1, Z-VAD-FMK, and Fer-1. RPL8 was also found to significantly alter the production of glycine, glutamate, and cysteine, upregulate glutathione synthase (GSS) protein levels, and enhance the GSH synthesis pathway.
Conclusions:
RPL8 plays a key role in regulating HCC ferroptosis and tumor progression, affecting cellular defense by regulating GSH synthesis. These insights highlight RPL8 as a promising target for therapeutic intervention in HCC.
Hepatocellular carcinoma (HCC) is significantly impacted by ferroptosis, a form of regulated cell death linked to iron metabolism and oxidative stress. This process is modulated by glutathione (GSH) levels. This study explored the role of ferroptosis-related genes (FRGs) in HCC and evaluated the impact of RPL8 on HCC progression and ferroptosis mechanisms.
Material and methods:
Through comprehensive TCGA-LIHC data analysis, we identified significant FRGs affecting HCC prognosis. The expression and functional significance of RPL8 in HCC were further evaluated using cell line models and tumor xenograft experiments, focusing on its effects on cell proliferation, invasion, migration, apoptosis, and regulation of ferroptosis.
Results:
Elevated RPL8 expression was observed in HCC tissues and cell lines. Functional experimental results showed that RPL8 regulation significantly affects cell proliferation, invasion, migration, and apoptosis. RPL8 silencing significantly inhibited tumor growth in tumor xenograft experiments. Furthermore, RPL8 knockdown resulted in increased 4-HNE levels, indicating increased lipid peroxidation and triggering ferroptosis, which was partially alleviated by inhibitors such as Nec-1, Z-VAD-FMK, and Fer-1. RPL8 was also found to significantly alter the production of glycine, glutamate, and cysteine, upregulate glutathione synthase (GSS) protein levels, and enhance the GSH synthesis pathway.
Conclusions:
RPL8 plays a key role in regulating HCC ferroptosis and tumor progression, affecting cellular defense by regulating GSH synthesis. These insights highlight RPL8 as a promising target for therapeutic intervention in HCC.
REFERENCES (45)
1.
Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol 2019; 71: 616-30.
2.
Kiani A, Uyumazturk B, Rajpurkar P, et al. Impact of a deep learning assistant on the histopathologic classification of liver cancer. NPJ Digit Med 2020; 3: 23.
3.
McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology 2021; 73 Suppl 1: 4-13.
4.
Sun VC, Sarna L. Symptom management in hepatocellular carcinoma. Clin J Oncol Nurs 2008; 12: 759-66.
5.
Chen Z, Xie H, Hu M, et al. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 2020; 10: 2993-3036.
6.
Vogt ACS, Arsiwala T, Mohsen M, Vogel M, Manolova V, Bachmann MF. On iron metabolism and its regulation. Int J Mol Sci 2021; 22: 4591.
7.
Kumar A, Sharma E, Marley A, Samaan MA, Brookes MJ. Iron deficiency anaemia: pathophysiology, assessment, practical management. BMJ Open Gastroenterol 2022; 9: e000759.
8.
Li Y, Song Y, Deng G, et al. Indoleamine 2, 3-dioxygenase 1 aggravates acetaminophen-induced acute liver failure by triggering excess nitroxidative stress and iron accumulation. Free Radical Bio Med 2021; 172: 578-89.
9.
Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res 2021; 31: 107-25.
10.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 2021; 22: 266-82.
11.
Averill-Bates DA. The antioxidant glutathione. Vitamins Hormones 2023; 121: 109-41.
12.
Asantewaa G, Harris IS. Glutathione and its precursors in cancer. Curr Opin Biotechnol 2021; 68: 292-9.
13.
de Souza I, Ramalho MCC, Guedes CB, et al. Ferroptosis modulation: potential therapeutic target for glioblastoma treatment. Int J Mol Sci 2022; 23: 6879.
14.
Sun T, Zhu W, Ru Q, Zheng Y. Silencing RPL8 inhibits the progression of hepatocellular carcinoma by down-regulating the mTORC1 signalling pathway. Human Cell 2023; 36: 725-37.
15.
Fan S, Zhang S, Kong D, et al. Integrative multi-omics analysis of identified ferroptosis-marker RPL8 as a candidate oncogene correlates with poor prognosis and immune infiltration in liver cancer. Comb Chem High Throughput Screen 2023; 26: 1298-310.
16.
Su LJ, Zhang JH, Gomez H, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev 2019; 2019: 5080843.
17.
Maugard M, Vigneron PA, Bolaños JP, Bonvento G. l-Serine links metabolism with neurotransmission. Progress Neurobiol 2021; 197: 101896.
18.
Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Rad Biol Med 2020; 152: 175-85.
19.
Kaiser K, Mallick R, Butt Z, Mulcahy MF, Benson AB, Cella D. Important and relevant symptoms including pain concerns in hepatocellular carcinoma (HCC): a patient interview study. Support Care Cancer 2014; 22: 919-26.
20.
Mikoshiba N, Miyashita M, Sakai T, Tateishi R, Koike K. Depressive symptoms after treatment in hepatocellular carcinoma survivors: prevalence, determinants, and impact on health-related quality of life. Psychooncology 2013; 22: 2347-53.
21.
Schacherer D, Schoelmerich J, Zuber-Jerger I. [The diagnostic approach to hepatocellular carcinoma]. Z Gastroenterol 2007; 45: 1067-74.
22.
Kumari R, Sahu MK, Tripathy A, Uthansingh K, Behera M. Hepatocellular carcinoma treatment: hurdles, advances and prospects. Hepat Oncol 2018; 5: HEP08.
23.
Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 2019; 23: 101107.
24.
Yao F, Deng Y, Zhao Y, et al. A targetable LIFR-NF-B-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun 2021; 12: 7333.
25.
Gao R, Kalathur RK, Coto-Llerena M, et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med 2021; 13: e14351.
26.
Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and cancer. Ann Rev Nutr 2018; 38: 97-125.
27.
Torti SV, Torti FM. Iron: the cancer connection. Mol Aspects Med 2020; 75: 100860.
28.
Hino K, Yanatori I, Hara Y, Nishina S. Iron and liver cancer: an inseparable connection. FEBS J 2022; 289: 7810-29.
29.
Dayani PN, Bishop MC, Black K, Zeltzer PM. Desferoxamine (DFO)--mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J Neurooncol 2004; 67: 367-77.
30.
Lamy PJ, Durigova A, Jacot W. Iron homeostasis and anemia markers in early breast cancer. Clin Chim Acta 2014; 434: 34-40.
31.
Xu L, Yang G, Song B, et al. Ribosomal protein L8 regulates the expression and splicing pattern of genes associated with cancer-related pathways. Turk J Biol 2023; 47: 313-24.
32.
Assi T, Watson S, Samra B, et al. Targeting the VEGF pathway in osteosarcoma. Cells 2021; 10: 1240.
33.
Du X, Qi Z, Xu J, et al. Loss of GABARAPL1 confers ferroptosis resistance to cancer stem-like cells in hepatocellular carcinoma. Mol Oncol 2022; 16: 3703-19.
34.
Zhang Y, Zhang J, Chen X, Yang Z. Polymeric immunoglobulin receptor (PIGR) exerts oncogenic functions via activating ribosome pathway in hepatocellular carcinoma. Int J Med Sci 2021; 18: 364-71.
35.
Liu L, Pang J, Qin D, et al. Deubiquitinase OTUD5 as a novel protector against 4-HNE-triggered ferroptosis in myocardial ischemia/reperfusion injury. Adv Sci 2023; 10: 2301852.
36.
Perkovic MN, Jaganjac M, Milkovic L, et al. Relationship between 4-hydroxynonenal (4-HNE) as systemic biomarker of lipid peroxidation and metabolomic profiling of patients with prostate cancer. Biomolecules 2023; 13: 145.
37.
Wiernicki B, Dubois H, Tyurina YY, et al. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis 2020; 11: 922.
38.
Nakamura T, Naguro I, Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta 2019; 1863: 1398-409.
39.
Sun L, Dong H, Zhang W, et al. Lipid peroxidation, GSH depletion, and SLC7A11 inhibition are common causes of EMT and ferroptosis in A549 cells, but different in specific mechanisms. DNA Cell Biol 2021; 40: 172-83.
40.
Qiao Y, Liu G, Leng C, et al. Metabolic profiles of cysteine, methionine, glutamate, glutamine, arginine, aspartate, asparagine, alanine and glutathione in Streptococcus thermophilus during pH-controlled batch fermentations. Sci Rep 2018; 8: 12441.
41.
Anchordoquy JP, Lizarraga RM, Anchordoquy JM, et al. Effect of cysteine, glutamate and glycine supplementation to in vitro fertilization medium during bovine early embryo development. Reprod Biol 2019; 19: 349-55.
42.
Xie S, Tian L, Niu J, Liang G, Liu Y. Effect of N-acetyl cysteine and glycine supplementation on growth performance, glutathione synthesis, and antioxidative ability of grass carp, Ctenopharyngodon idella. Fish Physiol Biochem 2017; 43: 1011-20.
43.
Patten DA, McGuirk S, Anilkumar U, et al. Altered mitochondrial fusion drives defensive glutathione synthesis in cells able to switch to glycolytic ATP production. Biochim Biophys Acta 2021; 1868: 118854.
44.
Wu Y, Wang D, Lou Y, et al. Regulatory mechanism of -hederin upon cisplatin sensibility in NSCLC at safe dose by destroying GSS/GSH/GPX2 axis–mediated glutathione oxidation-reduction system. Biomed Pharmacother 2022; 150: 112927.
45.
Kimura Y, Koike S, Shibuya N, Lefer D, Ogasawara Y, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine-and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci Rep 2017; 7: 10459.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.