Introduction
Adhesions are nonanatomic connections of fibrous tissue between organs and peritoneal surfaces [1]. Intra-abdominal adhesions may be classified as either acquired or congenital [2]. The formation of acquired adhesions is associated with an inflammatory response and extracellular matrix deposition in response to injury (surgical intervention, foreign bodies, blunt or penetrating trauma) [3, 4].
A congenital adhesion band is an intraperitoneal adhesion that has no relation to an intra-abdominal process (previous surgery, inflammatory diseases, peritonitis, etc.) [5]. Congenital intra-abdominal adhesions are a consequence of abnormal embryological development of the peritoneal cavity [2]. The regulatory role of cytokines and extracellular matrix remodeling factors in congenital intra-abdominal adhesions has not yet been defined.
Tumor necrosis factor (TNF) was originally described as a circulating factor that can cause necrosis of tumors [6]. Currently it is known that TNF-α is an acute-phase reactant, which leads to a systemic reaction including inflammation, fever, and activation of the complement and clotting cascades [7]. Higher levels of TNF-α are associated with higher concentration of interleukin-4 (IL-4) [8]. IL-4 has been viewed as the key cytokine of T helper (Th) type 2 differentiation. Th2 cells regulate tissue repair through “alternative” macrophage activation [9]. TNF-α is produced by activated monocytes, macrophages, and lymphocytes and elevated levels of TNF-α were detected in the case of pelvic adhesions [10]. TNF-α may be a good biological marker for postoperative peritoneal adhesion formation [11]. Early elevation of TNF-α concentrations in peritoneal fluid correlated with adhesion formation in rats [12].
Protein gene product 9.5 (PGP 9.5), also known as ubiquitin carboxyl-terminal hydrolase-1, was first detected as a “brain-specific protein” localized in neurons and neuroendocrine cells [13, 14]. PGP 9.5 expression has been subsequently documented in gonads, melanocytes, Merkel cells, dermal fibroblasts [15] and in some cells under certain conditions, such as fibroblasts during wound healing [16]. The ubiquitin system is central to the regulation of almost all cellular processes because it controls protein activity. PGP 9.5 is essential for the maintenance of axonal integrity [16].
Matrix metalloproteinases (MMPs) are a 24-member family of endopeptidases, which are involved in the degradation of extracellular matrix proteins [17]. Expression of most of the MMPs is low in normal tissues and increases when extracellular matrix remodeling is required [18].
Matrix metalloproteinase-2 (MMP-2) or 72 kDa gelatinase is a zinc-containing enzyme with a wide range of substrate specificity including type IV collagen, proteoglycans, fibronectin, and elastin [18, 19]. MMP-2 regulates endothelial cell proliferation, differentiation and vascular stabilization [20]. Endothelial cells have been reported to produce MMP-2 and store this enzyme in cytoplasmic secretory granules [21]. MMPs are produced by peritoneal mesothelial cells, adhesion fibroblasts, macrophages, neutrophils, and eosinophils in response to inflammation [18].
An important regulatory mechanism for the activity of MMPs is the proteolytic inhibition of MMPs by tissue inhibitors of metalloproteinases (TIMPs) [22]. The human TIMP family consists of four members, designated TIMP-1, -2, -3, and -4 [23]. The balance between MMPs and TIMPs is responsible for ECM proteolysis and a shift in favor of MMPs results in increased ECM proteolysis, whereas a shift in favor of TIMPs results in protection of the ECM and decreased proteolysis [24].
TIMP-2 has multiple effects on cell growth, apoptosis, and differentiation in addition to its inhibitory activity towards MMPs [25]. TIMP-2 inhibits MMP-2 activity, but it is also required for activation of pro-MMP-2 by selective interaction with membrane type 1-MMP (MMP-14) [24, 26]. TIMP-2 potently inhibits angiogenic responses induced by either basic fibroblast growth factor or vascular endothelial growth factor [27] and plays an important role in the stabilization of capillary networks during angiogenesis [28]. TIMP-2 expression has been documented in most cell types [26].
The aim of this study was therefore to investigate the presence and relative distribution of TNF-α, PGP 9.5, MMP-2 and TIMP-2 in specimens of patients with congenital intra-abdominal adhesions.
Material and methods
The experimental group material was obtained from 50 patients (23 males, 27 females) who underwent abdominal surgery due to complete or partial bowel obstruction (Table I). The control group was obtained from 8 patients (6 males, 2 females) with surgical repair of inguinal hernia (Table II). All patients were under 1 year of age. The study was performed according to the principles of the Declaration of Helsinki and was approved by the local ethics committee.
Table I
Experimental group
The tissue fragments were fixed for 24 h in a mixture consisting of 2% formaldehyde and 0.2 % picric acid in 0.1 M phosphate buffer (pH 7.2). These were then washed for 12 h in phosphate buffer (pH 7). Then tissues were embedded in paraffin and the blocks of paraffinized tissues were sectioned into slides 3–4 μm in thickness by means of a microtome. To obtain an overview picture, the slides were processed for hematoxylin and eosin.
Immunohistochemical analysis
Immunohistochemical methods [29] were applied for detection of signaling protein TNF-α and specific proteolytic enzymes. The following primary antibodies were used in immunohistochemistry: TNF-α (sc-52250, mouse, monoclonal, working dilution 1 : 100, Santa Cruz Biotechnology, Inc., USA), PGP 9.5 (439273A, rabbit, working dilution 1 : 100, ZYMED Laboratories, Invitrogen), MMP-2 (orb11061, rabbit, polyclonal, working dilution 1 : 100, Biorbyt Ltd., United Kingdom), TIMP-2 (sc-21735, mouse, monoclonal, working dilution 1 : 50, Santa Cruz Biotechnology, Inc., USA).
Adhesion tissue was deparaffinized and washed in alcohol and water, then washed for 10 min in wash buffer (Tri-buffered saline) and put for 5 min in EDTA boiling buffer in a microwave. When the samples had cooled down, they were washed twice for 5 min in wash buffer. Further, blocking for 10 min in 3% peroxide solution was performed, followed by washing twice for 5 min in wash buffer. To decrease background staining, normal blocking serum was used for 20 min. All tissue samples were incubated with primary antibodies for 1 h.
The specimens were incubated for 10 min at room temperature with HiDef Detection Amplifier (Mouse & Rabbit; REF 954D-31, LOT 1518304D, Cell Marque, USA). Another wash for 5 min in wash buffer was performed. Further, incubation for 10 min at room temperature with HiDef Detection HRP Polymer Detector (REF 954D-32, LOT 1518305D, Cell Marque, USA) was performed. It was followed by 5 min washing in wash buffer and 10 min processing with the DAB substrate-chromogen system (REF 957D-61, LOT 1423903E, Cell Marque, USA) to obtain positive structure staining in brown color. Samples were then rinsed in running water and stained with hematoxylin.
The presence of immunohistochemical markers in tissues was assessed according to the semiquantitative counting method [30]. The designations were as follows:
0, no positive structures found in the visual field,
0/+, occasionally marked structures in the visual field,
+, a few positive structures in the visual field,
+/++ few to moderate positive structures in the visual field,
++, a moderate number of marked structures in the visual field,
++/+++, moderate to large number of positive structures in the visual field,
+++, a large number of marked structures in the visual field,
++++, abundance of marked structures found in the visual field.
Statistical analysis
To characterize the research group descriptive statistic methods were used. Non-parametric statistics were applied and Spearman’s rank correlation coefficient (rs) was calculated to evaluate the correlation between proteins. The correlation was considered weak if the value of rs was 0–0.3, moderate if the value of rs was 0.31–0.69, and strong if the value of rs was 0.7–1. For the comparison of groups, the Mann-Whitney U-test was used. Two-tailed p-values of < 0.05 were considered as statistically significant. Data analysis was conducted using SPSS version 23.0.
Results
In most specimens, a moderate number of TNF-α positive structures was observed, but there was no statistically significant difference between the groups (U = 124.0, p = 0.082). A positive reaction for TNF-α was found in macrophages and fibroblasts (Figure 1 A). In 2 cases, the number of marked cells was moderate to large (++/+++), in 27 cases moderate (++), in 15 cases few to moderate (+/++). Only two specimens showed few (+) or occasional (0/+) TNF-α positive structures. Using the Spearman’s correlation test positive correlations were observed between the immunoreactive structures for TNF-α and PGP 9.5 (rs = 0.429, p = 0.003), TNF-α and TIMP-2 (rs = 0.588, p < 0.001). In the control group tissues, all cases showed a moderate (++) number of TNF-α containing macrophages and fibroblasts (Figure 2 A).
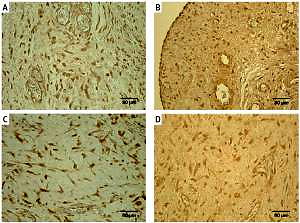
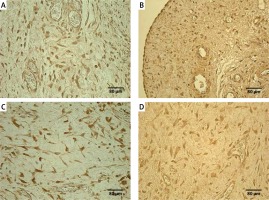
Figure 1
Immunoreactive structures in congenital adhesions. A – Moderate TNF-α positive fibroblasts and macrophages in congenital adhesions of a 36-day-old patient (TNF-α IMH, 250×). B – Moderate PGP 9.5 positive nerve fibers and fibroblasts in congenital adhesions of a two-day-old patient (PGP 9.5 IMH, 250×). C – Numerous MMP-2 positive macrophages and epithelioid cells in congenital adhesions of a 2-day-old patient (MMP-2 IMH, 250×). D – Moderate TIMP-2 positive fibroblasts, macrophages and endotheliocytes in congenital adhesions of a 56-dayold patient (TIMP-2 IMH, 250×)
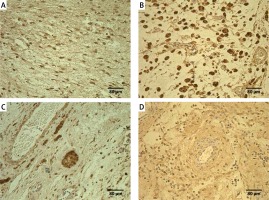
Figure 2
Immunoreactive structures in control group. A – Moderate TNF-α positive fibroblasts and macrophages of a 76-day-old patient in control group (TNF-α IMH, 250×). B – Moderate to numerous PGP 9.5 positive fibroblasts of a 76-day-old patient in control group (PGP 9.5 IMH, 250×). C – Moderate MMP-2 positive mesotheliocytes, endotheliocytes, fibroblasts and macrophages of a 53-day-old patient in control group (MMP-2 IMH, 250×). D – Moderate TIMP-2 positive fibroblasts and endotheliocytes of a 76-day-old patient in control group (IL-7 IMH, 250×)
A positive reaction for PGP 9.5 was observed in nerve fibers and shape modified fibroblasts. Most PGP 9.5 positive nerve fibers appeared around blood vessels. Some were found in the wall of the blood vessels (Figure 1 B). In the experimental group, PGP 9.5 positive structures were mostly seen in few to moderate (+/++) and moderate (++) numbers. In six specimens, the number of marked structures was moderate to large (++/+++), but in another 7 cases only a few (+) positive structures were seen. In control group tissues, positive structures were seen in significantly higher counts for PGP 9.5 (U = 58,5; p = 0,001). The control group tissues showed moderate (++) or moderate to large (++/+++) numbers of PGP 9.5 positive nerve fibers, fibroblasts and mesotheliocytes (Figure 2 B).
In the experimental group, few to moderate numbers of MMP-2 positive macrophages, epithelioid cells, neutrophils, fibroblasts and endotheliocytes were detected (Figure 1 C). In 20 cases, the number of marked cells was moderate (++), in 14 cases few to moderate (+/++), in another 14 cases few (+). In control group tissues, also MMP-2 positive fibroblasts, macrophages, endotheliocytes and mesotheliocytes (Figure 2 C) were found in few to moderate counts and there was no statistically significant difference between the groups (U = 174.0, p = 0.654).
In the experimental group tissues a positive reaction for TIMP-2 was seen in fibroblasts, macrophages and endotheliocytes (Figure 1 D). In 2 cases, the number of marked cells was moderate to large (++/+++), in 24 cases moderate (++), but in 14 few to moderate (+/++). Few (+) positive cells were observed in 8 cases. In control group tissues, positive structures were found in significantly higher counts for TIMP-2 (U = 112.0, p = 0.036). The most distinct difference was detected between TIMP-2 positive fibroblasts (U = 108.0, p = 0.022) and endotheliocytes (U = 34.0, p < 0.001) (Figure 2 D).
All semiquantitative results are summarized in Table III.
Table III
Semi-quantitative evaluation of immunoreactive structures
| Parameter | Experimental group | Control group | U | P-value | |
|---|---|---|---|---|---|
| TNF-α | Median | +/++ | ++ | 124.0 | 0.082 |
| IQR | 0/+ | 0 | |||
| PGP 9.5 | Median | ++ | ++/+++ | 58.5 | 0.001 |
| IQR | 0/+ | 0/+ | |||
| MMP-2 | Median | +/++ | +/++ | 174.0 | 0.654 |
| IQR | + | 0/+ to + | |||
| TIMP-2 | Median | ++ | ++ | 112.0 | 0.036 |
| IQR | 0/+ | 0 | |||
[i] TNF-α – tumor necrosis factor α, PGP 9.5 – protein gene product 9.5, MMP-2 – matrix metalloproteinase-2, TIMP-2 – tissue inhibitor of metalloproteinase-2, IQR – interquartile range, U – Mann-Whitney U value. Quantification of structures: 0 – no positive structures found in the visual field, 0/+ – occasionally marked structures in the visual field, + – a few positive structures in the visual field, +/++ – few to moderate positive structures in the visual field, ++ – a moderate number of marked structures in the visual field, ++/+++ – moderate to large number of positive structures in the visual field.
Using Spearman’s correlation test positive correlations were observed between the immunoreactive structures for MMP-2 and TIMP-2 (rs = 0.489, p < 0.001), MMP-2 and PGP 9.5 (rs = 0.515, p < 0.001) as well as between TIMP-2 and PGP 9.5 (rs = 0.524, p < 0.001).
Discussion
TNF-α and other cytokines have been studied for their potential role in acquired adhesion formation. Cytokines secreted by macrophages at the site of peritoneal injury play an integral role in regulating coagulation and fibrin formation, thereby influencing the development of adhesions [7]. Hypoxia causes normal peritoneal fibroblasts to acquire an adhesion phenotype, which exhibited significantly higher levels of TNF-α compared to normal peritoneal fibroblasts [7]. In our study, positive macrophages and fibroblasts for TNF-α were seen in moderate numbers in the examined specimens. Thus, inflammation is considered to be involved in the pathogenesis of congenital intraabdominal adhesions.
The role of TNF-α in the pathogenesis of postoperative adhesion formation has been proven by macroscopic and histopathologic evaluation of a rat model after treatment with TNF-α inhibitors. Decreased level of TNF-α was associated with a reduction in adhesion formation [31]. Wei et al. described resveratrol as an effective agent for the prevention of postoperative intraabdominal adhesions in rats. Resveratrol may inhibit the release of inflammatory factors that contribute to abdominal adhesion formation in abdominal injury, preventing extracellular matrix deposition and fibrosis [32]. In another study, early blocking of the activity of TNF-α after caecal abrasion in a rat model resulted in lower rates of adhesion formation macroscopically; however, according to the histological findings, there were no statistically significant differences between the groups [33]. Despite the lack of statistically significant difference between our study groups, predominantly moderate presence of TNF-α in the experimental group specimens suggests that TNF-α reduction may affect the development of congenital intraabdominal adhesions.
PGP 9.5 is widely used as a marker for innervation and neuropeptide containing structures. Innervation of adhesions has been studied little; therefore available data are limited and the role of PGP 9.5 in the case of congenital intraabdominal adhesions is unclear. Currently available data indicate that abundant PGP 9.5 expression is characteristic for fibrosis associated with human myofibroblasts and, as such, is likely to contribute to the fibrogenic process. Myofibroblast-like cells also stained positive for PGP 9.5 in fibrotic tissue of diseased livers [34].
Our results demonstrated moderate to numerous positive nerve fibers in isolated cases, but generally few to moderate PGP 9.5 positive structures in the tissue specimen. PGP 9.5 is necessary for the maintenance of axonal health and stability and its loss results in aggregation of ubiquitinated proteins, promotes axonal degeneration and neuronal death [16, 35]. Down-regulation of PGP 9.5 was observed in ischemic injury [35]. Therefore it is not possible to rule out PGP 9.5 down-regulation in congenital intraabdominal adhesions due to hypoxia induced damage.
The positive correlation between immunoreactive structures for TNF-α and PGP 9.5 suggests that nerve in-growth into the intraabdominal adhesions is induced by TNF-α and PGP 9.5 has a role in maintaining inflammation.
The local expression of MMPs and TIMPs has not been widely studied in intraabdominal adhesions. In order to evaluate tissue degeneration and remodeling in congenital intraabdominal adhesions we stained for MMP-2 and TIMP-2. While MMP-2 is thought to have a role in degrading the extracellular matrix, increased MMP-2 activity has also been associated with increased fibrosis [24]. Preliminary studies showed that serum levels of MMP-2 may be a useful marker for detecting a subclinical adhesion formation process [18]. Elevated MMP-2 levels have been correlated with peritoneal fibrosis in response to injury, adhesions or infection [36]. Prolonged expression of MMP-2 coincides with an increase in vascular density, but MMP-2 deficiency results in vascular regression and instability [20]. In our study, we found variable immunohistochemical positivity for MMP-2, while in general few to moderate MMP-2 positive structures were detected. Even though there was no statistically significant difference between the groups, we observed increasing presence of MMP-2 positive fibroblasts and macrophages in the experimental group.
TIMPs have various biological activities including modulation of cell proliferation, cell migration, anti-angiogenesis, anti- and pro-apoptosis and synaptic plasticity [28]. Balance between the expression of MMPs and TIMPs appears to be critical in normal wound healing, as alteration in their expression results in impaired wound healing and excess tissue formation such as development of peritoneal adhesion [37]. Imbalance between MMPs and TIMPs has been implicated in pathologic fibrosing diseases [25]. TIMP-2 plays a dual role in the regulation of MMP-2 activation: at low concentrations, it activates proMMP-2, whereas at higher concentrations, it completely inhibits this reaction [25].
Our study reveals moderate levels of TIMP-2. In the adhesion group, compared with relatively healthy controls, a pronounced decrease of TIMP-2 was observed in endotheliocytes and fibroblasts. TIMP-2 deficiency may promote monocyte/macrophage invasion [38] and leads to increased collagen synthesis and deposition [25]. The present study shows that increased MMP-2 activity, decreased TIMP-2 protein expression level and significantly increased MMP-2/TIMP-2 ratio contributed to fibrosis [39]. The imbalance between TIMP-2 and MMP-2 may indicate peritoneal fibrosis as a response to congenital peritoneal adhesions.
In conclusion, the positive correlation between the immunoreactive structures for TNF-α and PGP 9.5 suggests that nerve in-growth into intraabdominal adhesions might be induced by TNF-α and PGP 9.5 could have a role in maintaining inflammation.
The down-regulation of PGP 9.5 suggests that pathogenesis of congenital intraabdominal adhesions may be related to hypoxia induced damage.
The imbalance between MMP-2 and TIMP-2 may prove tissue fibrosis as a response to congenital peritoneal adhesions.