Introduction
Empyema thoracis is a disease originally diagnosed and treated by Hippocrates about 2,400 years ago. Through this disease, the pleural cavity is filled with pus, which is commonly caused by pneumonia [1, 2]. The mortality rate in this disease is as high as 15% [2]. Nowadays, reports show that about one million patients in the United States of America are hospitalized due to pneumonia, with 40% and 15% of them suffering from progressed pleural effusion and developed empyema thoracis, respectively [3].
The American Thoracic Society (ATS) has divided the evolution of pleural empyema into three stages. Stage I is the exudative phase. In stage II, mostly known as the fibrinopurulent phase, the effusion is converted to pus. However, thoracoscopy, video-assisted thoracoscopic surgery (VATS), and rarely open decortication are recommended to patients with loculations or peel. Finally, in stage III, pleura thickening with trapped lung may occur.
The existing surgical procedures for treating empyema thoracis in stages II and III can be either VATS or open decortication [4]. In 1918, the open decortication was performed by Graham and Bell for the first time and introduced for precise removal of the fibrous layer to allow lung re-expansion [2]. Also, VATS was standardized by Machinlay and Landrenea in 1988 [5, 6]. To treat empyema, both VATS and open decortication could be regarded as aggressive surgical approaches [4]. The mortality and morbidity rates after decortication were reported to be still close to 10% [2].
Today, the number of published articles in well-known journals is dramatically increasing and effectively defining the role of VATS in the treatment of empyema thoracis. However, the outcomes of both VATS and open decortication remain ambiguous.
Moreover, there is no randomized controlled trial (RCT) study in the literature [7–9]. In this regard, a common recognized approach to identify the advantages and disadvantages of two treatment procedures is to perform a meta-analysis to analyze the information extracted from the systematic reviews.
The subjects of the current systematic review and meta-analysis were selected based on Patients, Intervention, Comparison, and Outcomes (PICO) statements. The patients are those infected by empyema thoracis. The intervention includes two treatment procedures: (1) open thoracotomy decortication and (2) VATS decortication; finally, the patients’ answers to components defined in this study (i.e., postoperative prolonged air leakage, mortality, recurrence, failure or converted to thoracotomy, operating time, hospital stay, wound infection) are compared. However, academic researchers and surgeons still debate what the recommended treatment for effective management of empyema thoracis is [10].
Material and methods
Search strategy
A systematic search was carried out on Google Scholar, PubMed, and Scopus electronic databases from inception until March 27, 2017, in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11, 12]. To extract the relevant published papers a descriptive Boolean query was used: Query: Decortication AND Empyema AND (VATS OR video-assisted thoracoscopy surgery) AND ((open thoracotomy) OR (open surgery)).
For possible inclusion/exclusion of the articles in/from the study, the databases were searched using the “All Fields” option. Two anonymous investigators explored the extracted articles based on title, abstract, and full content where necessary.
Inclusion and exclusion criteria
All retrospective and cohort prospective studies in English including open thoracotomy surgery and VATS decortication surgery were considered for inclusion in this study.
All types of articles (i.e., full, original, review, abstract, epidemiologic studies, and meta-analysis) with unclear and inadequate data were excluded. All non-English published studies, except one abstract that had useful information, were excluded. The required data from the final eligible included studies were extracted into an Excel spreadsheet for further analyses.
The pooled analysis was performed using the odds ratio and standard mean difference (SMD) calculated for the studies of interest.
Meta-analysis process
The CMA version 2.2.064 [13] and Meta-MUMS tool were used. The Meta-MUMS tool, developed in MATLAB R2013a, provides an environment for carrying out the current meta-analysis with limited features including fixed-effects, random-effects meta-analyses, heterogeneity test, and publication bias. These analyses were done by calculating the odds ratio, log-odds ratio, and standard mean differences that are then presented as forest plots with high resolution. Heterogeneity was assessed by calculating Cochran’s Q and I2 [14]. Moreover, Egger’s test [15] was performed along with illustrative funnel plots in both fixed-effect and random-effect meta-analyses. Additionally, this tool was used to perform the meta-analysis within two groups using the term “data type” as dichotomous; i.e., it included events, mean, standard deviation, and sample size of each group [14]. These data were imported and exported as Excel files and illustrated as any type of image files.
Patients’ characteristics
The patients had thoracic empyema with positive clinical signs, confirmed by imaging techniques such as chest radiography, thoracic computed tomography (CT) scans, and ultrasonography. Adult patients with complicated parapneumonia and post-pneumonia in advanced stages II or III were considered for this purpose.
Surgery techniques
Video-assisted thoracoscopy decortication
This procedure could be performed for the patients for whom the chest tube drainage was a failure or when the lung did not re-expand after thoracentesis or tube thoracostomy. In the patients with thick pus or the presence of pleural thickening on the CT scan, and loculated fluid and debris, VATS was the recommended treatment procedure. After breaking down the loculations and lavaging the pleural space extensively, chest tubes should be placed carefully. In complicated types of stage II and special situations of stage III, the empyema thoracis was also treated by VATS. Any VATS failures, which mostly could happen at the late stages of empyema, were converted to open thoracotomy decortication.
Open thoracotomy decortication
Open thoracotomy decortication could be performed in almost all complicated types of stages II, III, radical, and VATS failure in treating empyema thoracis. This technique has been applied for the removal of fibrous tissue and peel exclusively from the parietal and visceral pleura to improve the lung re-expansion. Decortication relies on lung elasticity to fill the cavity and significantly improve the vital capacity, and lung perfusion and ventilation.
Outcome measures
Clinical outcomes were assessed by the disappearance of pleural fluid and full expansion of the affected lung. The postoperative prolonged air leakage, mortality, recurrence, failure (i.e., unsuccessful treatment of either open thoracotomy or VATS decortications) or converted procedures, postoperative hospital stay, times of operations, and wound infection were considered as clinical outcomes for both treatment procedures.
The reported postoperative complications include postoperative prolonged air leakage, wound infection, pleural space operation, blood transfusion, deep vein thrombosis, chylothorax, diaphragmatic lesion, atrial fibrillation, pneumonia, seroma, subcutaneous emphysema, intensive care unit (ICU) stay, thoracostomy drainage, morbidity, pain, paresthesia, bleeding after operation, myocardial infarction, cholecystitis, atelectasis, acute respiratory distress syndrome (ARDS), reintubation, tracheostomy, other pulmonary complication, ventricular arrhythmia, other hematologic complication, urinary tract infection, acute renal failure, other medical complication, other surgical complication, readmission, postoperative complication, atrial arrhythmia, bronchopneumonia, and ventilator dependence/support.
The reasons for conversion were technical inability, incomplete decortication, massive bleeding during operation, and life-threatening trauma to the adjacent organs such as great vessels.
Statistical analysis
For pooling the results from the studies of interest, a random-effects model was used. To illustrate the pooled results graphically, forest plots were used. Two heterogeneity indices – the Cochran Q test with a p < 0.05 and the I² index (percentage of variation across studies) – were used [14]. After generating the funnel plots and performing the required regression modeling such as intercept of Egger’s regression, and the p-values, the publication bias was assessed [15]. Based on various studies for assessing the publication bias, p-values less than 0.05 were regarded as significant [15–18]. The statistical analysis of all data was performed using both Meta-MUMS and CMA version 2.2.0.064 [13]. However, as the same results were obtained in terms of values and patterns of illustrations, only those for the Meta-MUMS tool (i.e., the implemented tool) will be demonstrated and discussed.
In the presence of heterogeneity, mixed-effects meta-regression for sample size difference, published year, hospital stay difference, latitude, and longitude was performed.
To explain the variance between studies, subgroup analysis was performed based on sample size (ideal = subgroup A and non-ideal = subgroup B) (a sample size is regarded as ideal if the sample size is more than 30 and the sample size difference is less than 63; otherwise the sample size is non-ideal), continent (America, Asia, Europe), and published year (Before 2010 = subgroup A and after 2010 = subgroup B).
The trim and fill method is a nonparametric and simple funnel plot-based tool used in meta-analysis for determining and adjusting the publication bias. In this methodology (also implemented in Meta-MUMS), the number of missing or unpublished studies that needs to be present in the meta-analysis is predicted along with their effects on the outcome [19, 20].
Results
Characteristics of studies
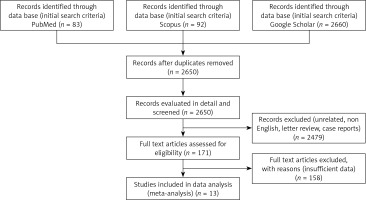
In this work, 2835 potentially relevant studies were identified and retrieved from the initial search of databases including Google Scholar, Scopus, and PubMed (Figure 1). After removing duplicates and applying the inclusion and exclusion criteria, only 13 articles remained. These studies included a total of 2219 patients treated with VATS and open thoracotomy decortication. Out of these, 1624 patients were treated with VATS, and 252 of these were failures and were converted. Additionally, 1099 patients were treated with open thoracotomy decortication (252 patients were converted and included).
Figure 1
PRISMA flow diagram for illustrating the flow of article selection in the systematic review for the metaanalysis procedure
Of 13 studies, 3 took place in the United Kingdom, 2 in the United States of America, 1 in China, 1 in Taiwan, 1 in Saudi Arabia, 1 in South Korea, 1 in Turkey, 1 in Italy, 1 in Brazil, and 1 in Switzerland.
One study [1] was prospective and the remaining ones were retrospective studies in nature [4, 21–31]. Tables I and II summarize the characteristics, demographics, type of procedures, outcomes, hospital stays, and times of operations for two surgical treatment procedures. Moreover, as all of the postoperative complications cannot be considered as one group, they have been considered as separate groups as postoperative prolonged air leakage, wound infection, pleural space operation, blood transfusion, deep vein thrombosis, chylothorax, diaphragmatic lesion, atrial fibrillation, pneumonia, seroma, subcutaneous emphysema, ICU stay, thoracostomy drainage, morbidity, pain, paresthesia, bleeding after operation, myocardial infarction, cholecystitis, atelectasis, ARDS, reintubation, tracheostomy, other pulmonary complication, ventricular arrhythmia, other hematologic complication, urinary tract infection, acute renal failure, other medical complication, other surgical complication, readmission, postoperative complication, atrial arrhythmia, bronchopneumonia, and ventilator dependence/support. Among these, only two postoperative complication outcomes (i.e., prolonged air leakage and wound infection) include three studies reporting sufficient data. The remaining ones include only zero, one, or two studies (Table III). Also, as it is not possible to conduct a meta-analysis and meta-regression with less than three studies and subgroup analysis with three or less than three studies [32–34], so the meta-analysis and meta-regression were performed for only prolonged air leakage and wound infection where the list in Table III was excluded; and subgroup analyses were performed for none of the postoperative complication outcomes listed in Table III or for prolonged air leakage and wound infection. For both procedures, the results of random-effects meta-analysis for the seven outcomes will be presented later. Moreover, the results of random-effects meta-regression based on published year, sample size difference, mean hospital stay difference, latitude, and longitude will also be demonstrated. Finally, the subgroup analyses based on sample size, continents, and published year will be presented.
Table I
Demographic characteristics of patients in studies treated by open decortication procedure (n = 13 studies)
Table II
Demographic characteristics of patients in studies treated by VATS procedure (n = 13 studies)
Table III
Characteristics of excluded postoperative complications based on the 13 studies’ reports
Meta-analysis models
There were no significant differences in postoperative prolonged air leakage results of open thoracotomy decortication and VATS (Figure 2 A, Table IV). Moderate heterogeneity was detected among the included studies (Table V).
Table IV
Detailed meta-analysis model with 95% confidence interval between two procedures
Table V
Heterogeneity meta-analysis model
Figure 2
Forest plots using odds ratio for postoperative prolonged air leakage (A), mortality (B), recurrence (C), failure or converted operations (D) Forest plots using standard mean difference (SMD) for times of operation (E), hospital stay (F), and wound infection (G)

There were no significant differences in mortality results of open thoracotomy decortication and VATS (Figure 2 B, Table IV). Moderate heterogeneity was detected among the included studies (Table V).
Also, there were no significant differences in recurrence results of open thoracotomy decortication and VATS (Figure 2 C, Table IV). Substantial heterogeneity was detected among the included studies (Table V).
The failure and conversion rate was significantly higher in VATS compared to that of open thoracotomy decortication (Figure 2 D, Table IV). The studies show a significant degree of heterogeneity (Table V).
Mean times of operations in open thoracotomy decortication were significantly longer than those of VATS (Figure 2 E, Table IV). Considerable, significant heterogeneity was detected among the included studies (Table V).
Postoperative hospital stays of open thoracotomy decortications were longer than those of VATS (Figure 2 F, Table IV). Considerable heterogeneity was detected among the included studies (Table V).
There were no significant differences in wound infection results of open thoracotomy decortication and VATS (Figure 2 G, Table IV). Moderate heterogeneity was detected among included studies (Table V), but it was not significant.
Meta-regression models
For the postoperative prolonged air leakage outcome (Figure 3 A, Table VI), the results of random-effects model meta-regression based on published year, latitude, sample size difference, and longitude showed that they cannot explain the heterogeneity of the included studies. However, mean hospital stay differences can explain 100% of heterogeneity (R² =100%). There were no relationships of the published year versus log odds ratio and latitude/longitude or sample size difference versus log odds ratio; however, a direct relationship was observed between mean hospital stay differences and log OR.
Table VI
Meta-regression model
Figure 3
Meta regression based on sample size, continent, latitude, longitude, and published year between two procedures for postoperative prolonged air leakage (A), mortality (B), recurrence (C), failure or converted operations (D), time of operation (E), and postoperative hospital stay (F)

For mortality outcome (Figure 3 B, Table VI), random-effects meta-regression on mean hospital stay differences was not carried out due to insufficient data. The results on published year, latitude, and sample size differences show that they cannot explain the heterogeneity of the included studies; however, the model based on longitude can explain 94% of heterogeneity (R² = 94%).
There were no relationships of published year versus log OR, latitude versus log OR, or sample size differences versus log OR, while there is a direct relation between longitude and log OR.
For recurrence outcome (Figure 3 C, Table VI) the data for random-effects met-regression based on hospital stay differences was not enough. Moreover, the results of random-effects meta-regression based on sample size difference can explain 100% of heterogeneity (R² = 100%) by an inverse relationship with recurrence. Moreover, the meta-regression models based on published year, latitude, and longitude cannot explain the heterogeneity as there were no relationships between log OR and the rest of the parameters.
For failure or converted operations outcome (Figure 3 D, Table VI), the data for hospital stay differences were not enough. The random-effects meta-regression based on published year, sample size difference, latitude and longitude cannot explain the heterogeneity. Moreover, there were no relationships between log OR and the other parameters. As the p-values were not significant.
For times of operations outcome (Figure 3 E, Table VI), the results of random-effects meta-regression based on published year, sample size difference, and longitude cannot explain the heterogeneity of studies according to the non-significant p value, and hence, there were no relationships between SMD of times of operations and the other parameters. Furthermore, random-effects meta-regression based on latitude can explain 47.6% of heterogeneity, suggesting a direct relationship between latitude and SMD of times of operations in the two procedures. Also, SMD of hospital stay can explain 77% of the heterogeneity, which shows a direct relation between SMD of hospital stay and times of operations in the two procedures.
For hospital stay outcome (Figure 3 F, Table VI), the results of random-effects meta-regression based on published year, sample size difference and longitude cannot explain the heterogeneity, since there was no relation between SMD of hospital stay in the two procedures and other parameters. Moreover, random-effects meta-regression based on latitude can only explain 5.29% of heterogeneity (R² = 5.29%), suggesting a direct relationship between latitude and SMD of hospital stay in the two procedures.
Additionally, for 13 studies, subgroup analysis and the heterogeneity subgroup analysis of the 7 outcomes based on sample size, continent, and published year were performed.
For wound infection (Table VI), the results of random-effects meta-regression based on published year, sample size difference, latitude, longitude, and mean hospital stay cannot explain the heterogeneity. Additionally, there were no relationships between log OR and the other parameters.
Subgroup analysis results
Subgroup analyses of postoperative prolonged air leakage outcome and wound infection as well as other remaining postoperative outcomes (Table III) between VATS and open decortication according to the sample size, the continents (i.e., America, Asia, and Europe), and the published year were not performed since the number of studies should be more than three [33].
Subgroup analyses of mortality outcome according to sample size and continent were not performed as the number of studies was less than 3 in these subgroups. Moreover, the subgroup analyses of the published year according to subgroup A, subgroup B, and overall show no differences in outcome of mortality when applying both treatment procedures (Figure 4 D). Heterogeneity subgroup analyses in subgroups A and B show substantial and no heterogeneity, respectively. Hence, the subgroup analysis of published year (Q between= 3.415, df = 1, p = 0.065) cannot explain the variance within the studies (listed in Tables VII and VIII).
Table VII
Subgroup analysis of mortality, recurrence, failure, operating time, postoperative hospital stay based on sample size, continent, and published year in the 13 studies
Table VIII
Heterogeneity subgroup analysis of mortality, recurrence, failure, operating time, postoperative hospital stay based on sample size, continent, and published year in 13 studies
Figure 4
Subgroup analyses of outcomes of studies based on sample size (A–C), published year (D–G), and continent (H, I)

Again, subgroup analyses of recurrence outcome according to sample size, continent, and published year were not performed since the number of studies in subgroups was less than 3.
The subgroup analyses of failure and converted operations outcome according to sample size in subgroup A, subgroup B, and overall show no, more, and more failure and converted operations for the VATS procedure, respectively (Figure 4 A). Moreover, heterogeneity subgroup analyses in subgroups A and B show considerable and substantial heterogeneity, respectively. Therefore, subgroup analysis of sample size (Qbetween = 0.248, df = 1, and p = 0.619) cannot explain the variance within the studies. The subgroup analyses according to the continent (i.e., America, Asia, and Europe), and overall show no, no, more, and more failure for the VATS procedure, respectively (Figure 4 H). The heterogeneity subgroup analyses according to the continents America, Asia, and Europe show no, no, and considerable heterogeneity, respectively. Thus, the subgroup analyses according to the continent (Qbetween = 4.647, df = 2, and p = 0.098) cannot explain the variance within the studies. Moreover, the subgroup analyses according to the published year in subgroup A, subgroup B, and overall show more, no, and more failure rates for the VATS procedure, respectively (Figure 4 E). The heterogeneity subgroup analyses in subgroups A and B show considerable and moderate heterogeneity, respectively. Thus, subgroup analyses according to published year (Qbetween = 1.697, df = 1, p = 0.193) cannot explain the variance within studies (listed in Tables VII and VIII).
The subgroup analyses of duration of operations according to sample size in subgroup A, subgroup B, and overall show no, more and more time of operations considering their SMD values for open thoracotomy decortication (Figure 4 B). The heterogeneity subgroup analyses in subgroups A and B show considerable and considerable amounts of heterogeneity, respectively. And hence, subgroup analysis of sample size (Qbetween = 3.207, df = 1, and p = 0.073) cannot explain the variance within studies (listed in Tables III and IV). Subgroup analyses according to the continent were not performed since the number of studies within the subgroups was less than 3. Moreover, the subgroup analyses according to the published year in subgroup A, subgroup B, and overall show more, no and more taken times of operations considering SMD values for open thoracotomy (Figure 4 F). The heterogeneity subgroup analyses according to subgroups A and B show considerable and considerable amounts of heterogeneity, respectively. And hence, the subgroup analyses of the published year (Qbetween = 0.765, df = 1, p = 0.382) cannot explain the variance within studies (listed in Tables VII and VIII).
Subgroup analyses of hospital stay according to sample size in subgroups A, B, and overall show no, more, and more differences considering their SMD for a hospital stay in open thoracotomy decortication versus VATS (Figure 4 C). Heterogeneity subgroup analyses in subgroups A and B show a considerable amount of heterogeneity. Subgroup analysis of sample size (Qbetween=6.683, df = 1, and p = 0.01) can explain R2 = 14.28% of variance within studies. Furthermore, the subgroup analyses of the continent show no, more, more, and more differences considering their SMD of hospital stay in open thoracotomy decortication compared to VATS (Figure 4 I). Moreover, heterogeneity subgroup analyses in America, Asia, and Europe show considerable heterogeneity for each. However, the subgroup analyses of the continent (Qbetween = 6.619, df = 2 and p = 0.037) can explain R2 = 19.12% of variance within studies. The subgroup analyses of the published year in subgroup A, subgroup B, and overall show more, no and more difference considering their SMD of hospital stay in open thoracotomy decortication compared to those of VATS (Figure 4 G). Heterogeneity subgroup analyses in subgroups A and B show a considerable amount of heterogeneity. Finally, subgroup analysis of published year (Qbetween = 1.517, df = 1, p = 0.218) cannot explain the variance within studies (listed in Tables VII and VIII).
Publication bias
The funnel plots of seven outcomes are illustrated (Figure 5). These outcomes used for identifying the publication bias are based on Egger’s regression test, which reveals no evidence for publication bias in two treatment procedures considering the outcomes except for the outcome of failure and converted operations (Table IX).
Table IX
Egger’s regression test for identifying the publication bias for seven outcomes
Figure 5
Funnel plots between two procedures for postoperative prolonged air leakage (A), mortality (B), recurrence (C), failure or converted operations (D), time of operation (E), hospital stay (F), and wound infection (G)

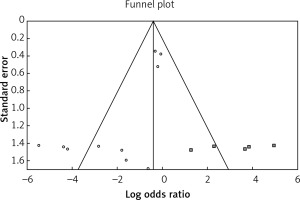
According to the identified publication bias, five imputable studies were found using the trim and fill method on the right side of the funnel plot (Figure 6) (odds ratio = 0.66637, lower limit = 0.24066, upper limit = 1.84512, Q = 67.84948, p = 0.4347). This result shows that despite adding these studies, there are no differences in the outcome of failure and conversion operations using VATS and open thoracotomy decortication. It has to be noted that 5 imputable studies in which open thoracotomy decortication was a failure were missed or not officially published or were selectively reported.
Discussion
The current systematic review and meta-analysis showed no promising trends toward the advantages of VATS in empyema thoracis. The results illustrated that the outcomes (i.e., hospital stay, and times of operations) of VATS are worse than those of open thoracotomy decortication. Generally, it is related to the time of performing anatomical opening and closing of the thoracotomy, which takes about 30–45 min. However, this period of time for opening and closing is omitted in the VATS procedure. It has also been shown that the rates of postoperative prolonged air leakage, wound infection, mortality and recurrence of either procedure had no advantage over the other. Moreover, although failures or converted operations in VATS were more frequent, due to the missing or selectively reported studies revealed by the trim and fill technique, it can be deduced that at least one of the procedures had no superiority over the other. The findings of this study indicated that VATS and open surgery decortication have an important place in empyema thoracis treatment and neither of them has advantages over the other.
Since Hippocrates’ day, open drainage of empyema has remained the only means of managing the late stages of empyema. Since that time, open thoracotomy decortication has been well accepted for definitive treatment of stages II and III of empyema [2].
Today, VATS is used as a very effective technique for treating stages II and III of empyema [1]. So, it is necessary to analyze the outcomes of VATS and open thoracotomy decortication using a systematic review and meta-analysis approach.
Today, there are several challenges to effectively treat empyema thoracis with both traditional and new approaches [23, 24, 28, 35, 36]. Some authors are in favor of performing VATS for treating empyema thoracis compared to open thoracotomy decortication [37].
Our literature review showed that there were only one systematic review and meta-analysis and no randomized controlled trial studies (RCTs) for comparing the VATS and open thoracotomy decortication [38]. Pan et al.’s study was performed on five studies, and they compared outcomes of VATS and open thoracotomy decortication. The results of hospital stay and operating time of VATS decortication are similar to ours with a lower mortality rate. Additionally, the postoperative prolonged air leakage of VATS decortication is less in Pan et al.’s study, while in the current study it has no advantages for both procedures. Recurrence outcome in the current study is less in both procedures, while Pan et al. reported only that of VATS decortication. Pan et al. did not report about the failure or conversion rate of VATS, while that rate in the current study for VATS is more than that of open thoracotomy decortication. Also, wound infection results of both procedures have no superiority over each other, while in the Pan et al. study, no information about this outcome was reported. Finally, they concluded that VATS decortication can be considered safe for selecting the first procedure in the management of empyema. Because of the small number of included studies in the Pan et al. meta-analysis, they found no heterogeneity in their study and hence meta-regression was not performed [39].
The current results should be considered along with the limitations of the included studies. Since designing and performing prospective RCT studies for the treatment of empyema according to ethical considerations is a difficult task, no RCTs were found to be included in this study.
The included studies were derived from PubMed, Scopus, and Google Scholar databases. Therefore, unpublished articles were not included since any systematic review might have unavoidable publication bias. In this study, publication bias was analyzed using graphical funnel plots and Egger’s regression test, where only one outcome with possible publication bias was seen. However, the trim and fill algorithm estimated that at least five potential studies have been missed.
Because of the retrospective nature of the study, surgeons with various individual surgical and treatment skills participated in included studies, which can influence the overall results. Surgeons’ bias is one of the limitations due to which many impactful studies may need to be excluded due to the absence of in-depth details for each patient such as treatment failure features [40].
So, a standard protocol for assessing the professionalism in surgeons is needed [41, 42]. Treatment selection bias is hard to assess and can be mostly eliminated in RCTs [43], and in the absence of RCTs, the propensity score matching is calculated [44]. However, it has been stated that “To date, there is no clear indication of whether propensity scores can remove the selection bias that jeopardizes quasi-experiments” [45]. Hence avoiding the surgeon bias has remained as a limitation; however, a portion of this can be determined through publication bias, which is mostly about selective reports.
For this purpose, it is suggested to report the outcomes by including the surgeons’ properties with respect to skills, years of surgery experience as well as educational background and facilities. Then, the surgeon bias can be evaluated using meta-regression and subgroup analyses. Additionally, the guideline of chest imaging for performing either of two procedures includes persistent pleural collections despite attempted drainage, restricted lung expansion, lung trapped by the pleural peel (it is described as “an inelastic membrane composed of fibroblasts that develops during the organization stage of a parapneumonic effusion and encases the lung, thus limiting its functional capability and resulting in trapped lung”. [46]), and multiloculation. However, there was no information on chest imaging, and hence the analysis for the selection of surgical approaches based on imaging was not feasible. Most of the time, the selection of surgical procedures depends on the estimated stages of empyema based on surgeons’ selection, which could be named surgeon’s bias as mentioned above. Insufficient data reported for the remaining postoperative complications (i.e., there were less than three studies that included those outcome measurements) is another limitation of this study which excludes them from further analysis.
Finally, to achieve reasonable results in these types of studies, performing more RCT studies with a sufficient sample size according to ethical issues is recommended for future systematic reviews and meta-analysis studies.
The current meta-analysis results confirmed that VATS is the best approach for reducing duration of hospital stay and time of operation with equivalent results for recurrence, postoperative prolonged air leakage, wound infection, and mortality in treating empyema thoracis.
Indeed, this meta-analysis may show that most often the VATS procedure was used at uncomplicated or stage I and stage II, or rarely at stage III of empyema; however, the results on the stages were cumulatively reported in the studies. On the other hand, open thoracotomy decortication was able to treat all stages consisting of complicated/uncomplicated forms of empyema thoracis and failure of VATS. Eventually, converted VATS decortication and its postoperative complications were finally treated by open surgery. Taking into account the results for the seven abovementioned outcomes, this study did not decrease the importance of open surgery.
Moreover, the results of the current study confirmed the American Association of Thoracic Surgery Guidelines (AATSG) for management of empyema in which VATS is presented as the first line approach in all patients with stage II acute empyema [47], and the European Association of Cardiothoracic Surgery expert consensus statement for surgical management of pleural empyema demonstrated benefits for surgical decortication by VATS at stages II and III of empyema, which are acceptable to undergo an operative procedure [48]. The only difference of this study is the presence of some limitations in performing the VATS procedure at stage III of empyema.
In conclusion, worldwide, the beneficial effects of VATS have been widely reported in treatment of early stages of empyema (i.e., stage II, with limited successful performance at stage III). The results of the current systematic review and meta-analysis suggest no major trends of superior outcomes with VATS versus open surgery decortication in the treatment of empyema thoracis. Hence, VATS and open thoracotomy decortication could be recommended in the treatment of empyema thoracis. However, failed or converted patients from VATS as well as those in advanced stages of empyema can be well managed by open thoracotomy decortication.