Introduction
Lungs are one of the organs most exposed to the external environment and more prone for infection. Many of the bacterial pathogens including both Gram positive and Gram negative strains and viruses cause respiratory infection which causes lung injury. Influenza A (H1N1) virus induces lung infection which results in pandemic outbreaks in humans [1]. Patients suffering from acute H1N1 infection may die due to lung injury and respiratory distress. The literature reveals that enhancement of the immunity of the host improves the host defense against the viral infection [2]. In viral lung infection the levels of chemokines and inflammatory cytokines and number of immune cells are enhanced in the lung tissue [3]. In patients suffering from H1N1 lung infection the mortality rate is high due to leukocyte and cytokine induced lung injury [4]. Toll-like receptors (TLRs) are present on immune cells which activate the expression of cytokines, phagocytosis and migration of immune cells by recognizing the pathogens [5]. TLR-7 is one the receptor of the TLR family which recognizes the single-stranded RNA (ssRNA) of the virus and which further leads to enhancement of macrophages and cytokines in the lung tissue by activating the MyD88 dependent pathway [6]. This all leads to the lung injury which could be a reason for higher mortality. In early stages of viral infection use of anti-viral drugs is avoided by physicians, which further leads to infectivity of the antiviral drug in H1N1 infection. Thus there is a need for development of an alternative medicine that could be used for the treatment of viral infection.
Toonaciliatin K is one of the limonoids, a secondary metabolite obtained from citrus fruits [7]. The literature reveals that limonoids possess hepatoprotective, anti-inflammatory, anti-obesity, and anticancer activity [8–11]. Toonaciliatin K shows anti-inflammatory properties in several models including arthritis [12]. The present investigation determines the protective effect of toonaciliatin K against lung injury induced by lung infection of H1N1 influenza virus.
Material and methods
Animals
Albino mice (age: 8–12 weeks; weight: 17–23 g) were used in the study. All the animals were procured from Jing Mei Company, China. Standard guidelines (humidity: 60 ±5%; temperature: 25 ±2°C) were used to maintain the animals with a 12 h light and dark cycle. Protocols of the investigation were approved by the ethics committee of Tongliao City Hospital, China (IAEC/TCH/2018/08). Influenza virus for mouse infection was provided by the Institute of Medicinal Biotechnology, China. H1N1 virus was administered to the mice at 10 LD50 intranasally.
Experiment
All the mice were separated into four different groups: normal group; infected group; toonaciliatin K 16.5 and 33 mg/kg, which received toonaciliatin K 16.5 and 33 mg/kg intragastrically 1 h after exposure to the H1N1 virus and thereafter treated for the duration of 2 weeks. Infection was induced by exposing the anesthetized mice to the H1N1 virus (10 LD50 in a volume of 30 ml) intranasally at day zero. At the end of the protocol, survival, body weight and clinical symptoms were observed in all mice.
Isolation of lung and estimation of lung index
All the animals were sacrificed by cervical dislocation and the lung was removed from each animal. The isolated lung was stored in 10% phosphate-buffered formalin. Weight of the isolated lung was estimated and lung edema was estimated by determining the lung index.
Histopathology study
The left lobe from the isolated lung was placed in liquid paraffin and a tissue section of 5 μm thickness was sectioned by microtome. Further H + E staining was used to stain the tissue and histopathological alteration was examined by using a trinocular microscope. Pathological score from the scale 0–3 of lung tissue was calculated as per the previously reported study.
Assessment of chemokines and cytokines
Isolated lung tissue was homogenized and levels of chemokines (MIP-1 and MCP-1) and cytokines (INF-α, TNF-α, IL-1 and IL-6) were estimated in the tissue homogenate as per the instructions given by the manufacturer of the ELISA kits.
Western blot assay
Protein samples were isolated from homogenized tissues and quantified using a bicinchoninic acid (BCA) assay kit. We used 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) to separate the proteins. Proteins were then transferred to a nitrocellulose membrane using an electroblotting technique. The membrane was blocked using a 5% blocking solution (non-fat milk) and then incubated in a blocking buffer with the following primary antibodies overnight at 4°C: TLR-7 (1 : 1000; Santa Cruz Biotechnology, USA), NF-κB p65 (1 : 200; Santa Cruz Biotechnology, USA), MyD88 (1 : 1000; Santa Cruz Biotechnology, USA) and GAPDH (1 : 1000; Cell Signaling Technology, USA). The following day, goat secondary antibody conjugated with horseradish peroxidase (HRP) was added to the blocking buffer (1 : 1,000, non-fat milk), and a chemiluminescence kit (Thermo Fisher Scientific, Shanghai, China) was used to detect the proteins. Chemiluminescence was used to enhance the blot and ImageLab software was used to perform the densitometric analysis of blots.
Reverse-transcription polymerase chain reaction (RT-PCR)
RNA was isolated from separated lung tissue using TRIzol Reagent (Thermo Fisher). The RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher) was used to reverse-transcribe the RNA. Specific primers were mixed with RT-2 SYBR green master mix to evaluate gene expression by RT-PCR. The procedure used for all samples was as follows: 98°C for 2 min, followed by 25–40 cycles of 98°C for 10 s, then 55°C for 5 s, and 72°C for 20 s. mRNA expression levels of MyD88, TLR-7, iNOS, NF-κB p65 and GAPDH were calculated according to relative standard curves, which were generated by plotting the quantification cycle (Cq) against the log amount of total cDNA added to the reaction. The 2–ΔΔCq method was used to estimate the expression of the relative target gene (Table I).
Table I
Primer sequence used in the RT-PCR
Statistical analysis
All data were expressed as mean ± SEM (n = 10). The statistical analysis was performed using one way ANOVA. Post-hoc comparison of means was carried out by Dunnett’s post hoc test (Version 6.1, GraphPad Prism Software, Inc., San Diego, CA, USA). The level of statistical significance was set at p < 0.05.
Results
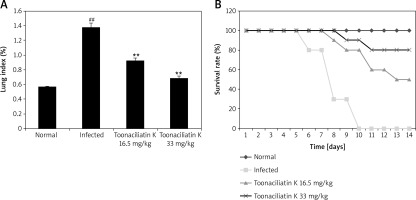
Effect of toonaciliatin K on the survival rate
Assessment of the effect of toonaciliatin K treatment on the survival rate and lung index of H1N1 infected lung injured mice is shown in Figure 1.It was observed that the survival rate of H1N1 infected mice was reduced compared to the normal group of mice. However, the survival rate was improved in the toonaciliatin K treated group compared to the infected group of mice in a dose-dependent manner. The lung index was found to be higher in the infected group compared to the normal group of mice. Treatment with toonaciliatin K alleviates the lung index in H1N1 infected lung injured mice.
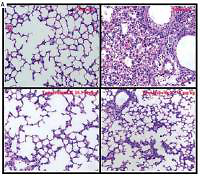
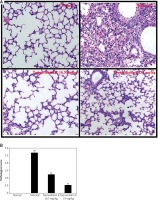
Effect of toonaciliatin K on the histopathology of lung tissue
Assessment of histopathology of lung tissue of toonaciliatin K treated H1N1 infected mice was done at the end of the protocol and the pathological score is shown in Figures 2 A, B. Transverse section (TS) of lung tissue of the infected group of mice show profuse hemorrhage, consolidation and edema too. However, histopathology of the toonaciliatin K treated group shows reduced edema, infiltration of neutrophils and thickness of the alveolar wall (Figure 2 A). Moreover, the pathological score was higher in the infected group than the normal group of mice. Treatment with toonaciliatin K ameliorates the pathological score of lung tissue of H1N1 infected mice (Figure 2 B).
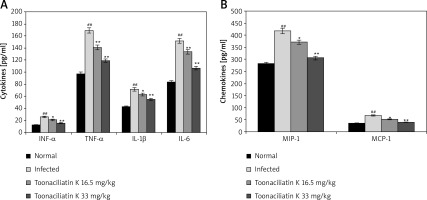
Effect of toonaciliatin K on cytokines and chemokines
Levels of chemokines and cytokines in the lung tissue homogenate of toonaciliatin K treated H1N1 infected lung injured mice are shown in Figure 3. It was observed that the levels of cytokines (INF-α, TNF-α, IL-1β and IL-6) and chemokines (MIP-1 and MCP-1) were significantly higher in the lung tissue homogenate of the infected group than the normal group of mice. There were lower levels of chemokines and cytokines in the lung tissue in the toonaciliatin K treated group than the infected group of mice in a dose dependent manner.
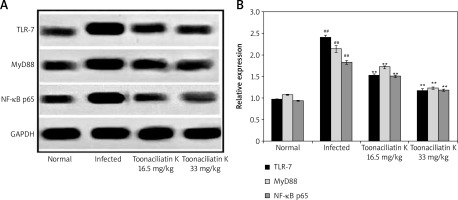
Effect of toonaciliatin K on expression of NF-κB p65, MyD88 and TLR-7
Data of western blot assay showed the expression of NF-κB p65, MyD88 and TLR-7 proteins in the lung tissue homogenate of toonaciliatin K treated H1N1 infected lung injured mice (Figure 4). Expression of NF-κB p65, MyD88 and TLR-7 proteins was higher in the lung tissue homogenate of the infected group compared to the normal group of mice. There was lower expression of NF-κB p65, MyD88 and TLR-7 proteins in the lung tissue homogenate of the toonaciliatin K treated group compared to the infected group of mice.
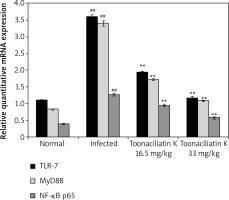
Effect of toonaciliatin K on mRNA expression of NF-κB p65, MyD88 and TLR-7
The effect of toonaciliatin K on the mRNA expression of NF-kB p65, MyD88 and TLR-7 in the lung tissue homogenate of H1N1 infected lung injured mice is shown in Figure 5. There was higher mRNA expression of NF-kB p65, MyD88 and TLR-7 in the lung tissue homogenate of the infected group than the normal group of mice. Treatment with toonaciliatin K ameliorates the mRNA expression of NF-kB p65, MyD88 and TLR-7 in the lung tissue homogenate of H1N1 infected lung injured mice.
Discussion
H1N1 virus induces lung infection which causes lung injury by altering the inflammation and immune cell infiltration in the lung [13]. Treatment of H1N1 infection with a conventional drug has several limitations and thus the present study determines the protective effect of toonaciliatin K on the H1N1 infected lung injured mice. The effect of toonaciliatin K was assessed by estimating the survival rate and lung edema by the lung index. Histopathological changes were determined by H + E staining and western blot and an RT-PCR study was also performed on the lung tissue homogenate.
The H1N1 infection induced mouse model is well reported and also shows the inflammation of lung tissue and higher immune response [14]. CD8(+) Ts cells are involved in the chronic immune response in several diseases such as multiple sclerosis, systemic lupus erythematosus, and systemic sclerosis [15]. Thus this study also induces lung infection of H1N1 virus in a mouse model. H1N1 infection is associated with high mortality. Data of the study also suggested the lower survival rate in the infected group and treatment with toonaciliatin K enhances the survival rate in a dose dependent manner. Moreover, toonaciliatin K treatment attenuates lung edema by reducing the lung index compared to the infected group of mice. The literature reveals that H1N1 infection leads to enhancement of the concentration of cytokines due to an excessive immune response [16]. Chemokines are activated due to viral infection enhancing the infiltration of leukocytes in the lung. Further macrophages and phagocytes secreted inflammatory cytokines which causes lung injury [17, 18]. Data of the investigation reveal that treatment with toonaciliatin K reduces the level of cytokines and chemokines in the lung tissue compared to the infected group of mice. Moreover, toonaciliatin K ameliorates the histopathological changes in the lung tissue of H1N1 infected lung injured mice.
TLR-7 is a receptor of the TLR family which recognizes the single-stranded RNA (ssRNA) of the virus and which further leads to enhancement of macrophages and cytokines in the lung tissue by activating the MyD88 dependent pathway which further activates NF-kB p65 [19]. Activation of NF-kB p65 enhances the inflammation and immune response by regulating the secretion of chemokines, interferon, cytokines and growth factors [20]. Inflammation in the lung tissue reduces the function of the lung, which leads to an increase in mortality [21, 22]. Results of the investigation reveal that the expression of TLR-7, Myd88 and NF-kB p65 was reduced in the lung tissue of the toonaciliatin K treated group compared to the infected group of mice.
In conclusion, data of the study suggested that toonaciliatin K protects against lung injury in lung H1N1 lung infection. Toonaciliatin K modulates immune cell function and inflammatory cytokines by regulating the TLR-7/Myd88/NF-kB p65 pathway in H1N1 induced lung injured mice.