Skin flap transplantation is an effective way to treat soft tissue defects and has been applied to fresh wounds and old trauma. Angiogenesis and revascularization are the major processes that affect the survival of a transplanted skin flap [1]. However, owing to the absence of anatomically named vascular vessels, blood supply in the flaps mainly relies on the microvascular network in the pedicle, which is often insufficient, leading to ischemic necrosis and unsatisfactory therapeutic efficacy. Therefore, it is of great significance to prevent and cure necrosis of the grafted flap resulting from ischemia due to impaired arterial inflow [2].
Tβ4 is a naturally occurring regenerative peptide with 43 amino acid residues. It has a potential to treat severe dermal injuries resulting in faster repair of chronic wounds [3]. Tβ4 has been demonstrated to have strong wound healing and potent anti-inflammatory activities in numerous models of corneal injury [4]. Although proven effective in increasing the survival of random skin flaps [5], more mechanisms related to this effect need to be elucidated.
The present study aimed to determine the effect of Tβ4 on the viability of rat random-pattern skin flaps and explore the possible mechanisms underlying the therapeutic activity.
Wistar rats, weighing 300–320 g and aged 2–3 months, were used to construct the McFarlane flaps (3 × 9 cm) containing the dartos and muscle [6] and the flaps were elevated. After the elevation, the flaps were sutured back in situ with 4-0 surgical nylon sutures. The flap models were intraperitoneally administered with 0 (control, saline), 2 and 10 mg/kg/day Tβ4 (recombinant human tβ4, cat. no. SRP3324, Sigma-Aldrich Chemical Company, USA) dissolved in saline at 50 mg/l for 7 days. At 3, 5, 7 and 9 days after surgery, the flaps were pictured using a digital camera and the survived areas on the flaps were measured using ImageJ software. The survival percentage was calculated by dividing the total flap area by the survived area × 100. The viability of flaps was assessed based on the color, formation of a scab, and vessel refilling. The flaps were considered necrotic if the surface of the flap was covered with blackened scabs with a hard texture and inelastic.
To assess vessel development, the skin samples at the center of the flaps were isolated and stained with hematoxylin and eosin (H&E) (Solarbio, Beijing, China). H&E-stained slices were then examined under a light microscope (Olympus BX-53, Japan) for vessel development, erythrocyte extravasation and other evidence of inflammatory reactions. To assess the level of microcirculation, mean vessel density (MVD) of flap tissue was determined by counting the vessels per unit area (/mm2) in six randomly selected microscopic fields. To assess gene expression, proteins in tissues taken from the center of the flaps were extracted and separated on 12% sodium dodecyl-sulfate polyacrylamide gel for electrophoresis (SDS-PAGE). After separation, proteins were blotted to PVDF (polyvinylidene difluoride) membranes and were reacted with antibodies against c-Myc, β-catenin, NF-κB, caspase-3, VEGF, and β-actin (used as an internal control) and detected with horseradish peroxidase-conjugated antibody to rabbit IgG. The intensity of blot signals was measured using ChemiDocXRS+ on an ultrasensitive chemiluminescence imaging system. All assays were conducted in triplicate in three independent experiments. One-way ANOVA (analysis of variance) was employed to compare means among multiple independent variables using SPSS version 19.0 (IBM Corporation, USA) for Windows (Microsoft Corporation, USA). A value of p < 0.05 was considered statistically significant.
This study was approved by the Ethics Committee of Wuxi 9th People’s Hospital Affiliated to Soochow University (SUAS-2312).
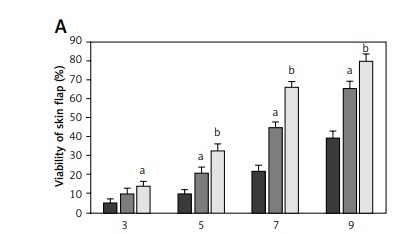
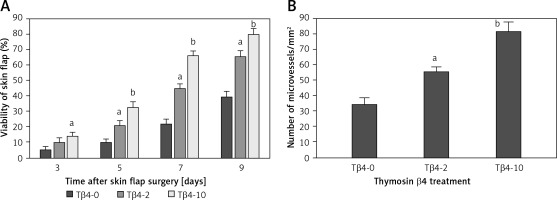
All rats survived the operation. After McFarlane flap model construction, the distal parts of flaps were slightly swollen and pale in color within 2 days after the surgery. Necrosis was observed around the flaps where the tissues became dark, dry, crumpled, and stiff. The survival areas of flap increased as the times after surgery increased, and the percentages of flap survival area were significantly higher in rats treated with 2 and 10 mg Tβ4 as compared to control rats (p < 0.05, Figure 1 A). On day 9 after surgery, biopsy was performed to examine angiogenesis in the flaps by counting vessel density after the tissues were stained with hematoxylin and eosin solution. Compared with control flaps, the flaps treated with 2 and 10 mg of Tβ4 had significantly greater MVD (p < 0.05, Figure 1 B).
Figure 1
Effect of thymosin β4 on the survival of rat random-pattern cutaneous flaps and expression of wounding-related genes. A – Viability of rat random-pattern cutaneous flaps after treatment with thymosin β4. McFarlane flap models of rat were constructed (n = 15) and treated with 0 to 10 mg of thymosin β4/kg/day for 7 days. The survival areas of flaps were determined based on photography and calculated as surviving area/total flap area (viability %). B – Mean microvessel density in rat random-pattern cutaneous flaps after treatment with thymosin β4. McFarlane flap models of rat were constructed (n = 15) and treated with 0 to 10 mg/kg/day for 7 days. The number of microvessels in the flap were determined based on H&E assessment. C. Expression of NF-κB (a) and VEGF (b) in rat random-pattern cutaneous flaps after treatment with thymosin β4. McFarlane flap models of rat were constructed (n = 15) and treated with 0 to 10 mg/kg/day for 7 days. NF-κB and VEGF in the flap were assessed using Western blot analysis. Upper panel: representative Western blots, lower panel: statistical analysis of proteins. D – Expression of caspase-3 (a), β-catenin (b) and c-Myc (c) in rat random-pattern cutaneous flaps after treatment with thymosin β4. McFarlane flap models of rat were constructed (n = 15) and treated with 0 to 10 mg/kg/day for 7 days. Caspase-3, β-catenin and c-Myc in the flap were assessed using Western blot analysis. Upper panel: representative Western blots, lower panel: statistical analysis of proteins. ANOVA was applied to compare means among groups. Columns labeled with different letters are significantly different (p < 0.05)
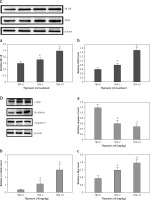
To assess the involvement of immune, inflammatory and angiogenetic responses, Western blot analysis was performed using the flap tissues and revealed that NF-κB, a regulator of various proinflammatory and anti-inflammatory factors, was significantly upregulated following Tβ4 treatment, particularly after 10 mg of Tβ4 (p < 0.05, Figure 1 C-a). Similar upregulation of VEGF was detected in Tβ4-treated flaps (p < 0.05, Figure 1 C-b). Since apoptosis is likely involved in surgery-induced skin damage, we also assessed the caspase-3 levels in the flap tissues. The data showed that there was significant content of caspase-3 9 days after the surgery. However, after Tβ4 treatment, caspase-3 levels were significantly lower (p < 0.05, Figure 1 D-a). Since the Wnt/β-catenin signaling pathway is highly conserved evolutionarily and plays a very important role in regulating cell proliferation, growth, differentiation and maintaining the stability of the intracellular environment, and β-catenin is the key regulator of this pathway, we assessed the level of β-catenin in the flaps. The data showed that β-catenin was significantly upregulated in the Tβ4-treated flaps as compared to the flaps not treated with Tβ4, and the upregulation again was Tβ4-concentration dependent (p < 0.05, Figure 1 D-b). As a downstream target gene, the c-Myc level was also upregulated significantly after Tβ4 treatment in a concentration-dependent manner (Figure 1 D-c, p < 0.05).
Our work showed that Tβ4 promotes the survival of skin flaps with increased angiogenesis, upregulates the levels of NF-κB, VEGF and β-catenin and reduces the level of caspase-3. It is likely that Tβ4 promotes the survival of skin flaps via activating the Wnt/β-catenin signaling pathway. Improving the survival of the transplanted flap is of great significance in reconstructive surgery. Necrosis due to blood supply disorder has been a major obstacle that hampers the clinical utilization of random-pattern skin flaps. Several mechanisms have been proposed to explain the necrosis, including I/R injury and inflammation [7]. Based on these mechanisms, inhibition of oxidative stress and inflammation has been investigated as possible treatment options, and improved skin flap survival was observed in rats after treatment with sumatriptan with anti-inflammatory effects and ivermectin that reduces I/R injury via the GABAergic pathway [8, 9]. As a lymphocyte growth factor, Tβ4 has strong wound healing and anti-inflammatory activities in numerous models of injury [4, 10]. In the present study, we found that Tβ4 improved the survival of random-pattern skin flaps in a dose-related way, further confirming the usefulness of Tβ4 for flap surgery [5]. The improvement is likely associated with enhanced angiogenesis in the flaps, where an increased number of microvessels and elevated VEGF expression were observed, suggesting that Tβ4 promotes angiogenesis via VEGF, as reported earlier [5]. Elevated NF-κB levels were observed in the flaps, particularly after Tβ4-10 treatment, indicating that Tβ4 is likely responsible for the regulated NF-κB expression. Apoptosis is likely involved in the injury induced during the flap surgery. Our assessment showed that caspase-3 protein levels were the highest in the flap tissue not receiving Tβ4 and gradually decreased as Tβ4 concentration increased, suggesting that Tβ4 might suppress apoptosis to accelerate the repair of damage and wound healing. A recent study showed that Tβ4 may inhibit apoptosis induced by I/R after severe scald injury by downregulating STAT1 and upregulating STAT3 expression [11].
The Wnt/β- catenin signal pathway is involved in regulating the proliferation, growth and differentiation of cells [12]. In our assessment, increased β-catenin levels in the flaps were observed following Tβ4 treatment, suggesting that Tβ4 may upregulate β-catenin expression and activate the Wnt/β-catenin signal pathway, consequently upregulating downstream targets such as c-Myc. A previous gain-of-function study showed that Wnt/β-catenin promotes wound closure in a self-controlled rat model with full-thickness skin wounds and c-Myc upregulation could enhance the differentiation of epidermal stem cells (ESCs) into keratinocytes [13]. Therefore, activation of Wnt/β-catenin and related upregulation of c-Myc and VEGF by Tβ4 are likely among the mechanisms underlying the improved flap survival.
The present study has some limitations that need to be addressed. For example, only two Tβ4 doses were applied; the optimal (dose) concentration of Tβ4 needs to be defined to maximize the therapeutic effect. Other pathways related to wound healing such as the SIRT1-mediated AMPK/mTOR signaling pathway should be profiled to gain more comprehensive understanding of the molecular mechanisms underlying the improved flap viability.