Introduction
The impact of cardiovascular diseases (CVDs) is estimated to have been US $906 billion in 2015 and is expected to rise by 22% during this decade [1]. Nowadays, it is well known that CVDs are the leading cause of mortality and remain one of the major causes of health loss for all regions of the world [2]. In particular, ischemic heart disease (IHD) and atherosclerosis are the main causes of premature death in Europe and are responsible for 42% of deaths in women and 38% in men under 75 years old [3].
Among the modifiable risk factors for CVDs, elevated total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) plasma concentrations are the most important ones for coronary heart disease (CHD) development, whereas high concentrations of plasma high-density lipoprotein cholesterol (HDL-C) in certain conditions are considered protective [4, 5]. A large body of clinical evidence has demonstrated that each further reduction of LDL-C by 1 mmol/l (~40 mg/dl) could decrease by about one-fifth the risk of coronary artery disease, revascularization and ischemic stroke, highlighting that a reduction of LDL-C of 3.2 mmol/l (125 mg/dl) could lead to a decrease in risk of about 40–50%, in absence of an increased risk of cancer or non-cardiovascular (CV)-related death [6]. However, a report from the American Heart Association (AHA) underlined that in the US only 46.6% of the pharmacologically untreated adults present targeted TC levels < 200 mg/dl [7], and the situation is similar in Western Europe [8]. Certainly, a reduction of 1 mmol/l of LDL-C is mostly achievable through lifestyle improvements associated with lipid-lowering nutraceuticals [9]. In this context, red yeast rice (RYR) is one of the nutraceuticals with the most solid clinical evidence of efficacy [10]. The main cholesterol-lowering putative mechanism of action of RYR is the reversible inhibition of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase (the key enzyme in endogenous cholesterol synthesis), mainly due to the presence of monacolins, and in particular of monacolin K (structurally homologous to lovastatin), which is the most lipid-lowering polyketide obtained during yeast fermentation [11]. The dose-dependent lipid-lowering efficacy of RYR has been confirmed by large meta-analyses of randomized controlled clinical trials (RCTs); however, its safety is also inversely related to the daily dose employed [12, 13]. For this reason, low-dose monacolin K should be preferred (and it is now recommended by the European Food Safety Agency (EFSA) with doses up to 3 mg), and its efficacy should be increased by co-administration with other nutraceuticals acting with a synergistic mechanism of action [14]. In this context, a dietary supplement more widely used in clinical practice is Armolipid Plus®, which contains low-dose RYR and berberine, which is a natural inhibitor of the proprotein convertase subtilisin/kexin type 9 (PCSK9) [15]. Moreover, Armolipid Plus® is the only combined lipid-lowering nutraceutical recommended by the International Lipid Expert Panel (ILEP) for the management of hypercholesterolemia in statin-intolerant patients [16].
The metabolic effect of Armolipid Plus® has been investigated and quantified in many studies, including a large meta-analysis of 12 RCTs including more than 1050 patients overall [15], which showed that supplementation for the mean time of 20 weeks was associated with significant improvements in body mass index (BMI = –0.25 kg/m2, p = 0.008), TC (–25.07 mg/dl, p < 0.001), LDL-C (–26.67 mg/dl, p < 0.001), HDL-C (+1.84 mg/dl, p < 0.001), triglycerides (–11.47 mg/dl, p < 0.001), high-sensitivity C-reactive protein (hs-CRP, –0.61 mg/l, p = 0.022), and fasting glucose (–3.52 mg/dl, p < 0.001), with a good tolerability profile. However, berberine is not available in all countries [17]. A possible alternative could be the use of artichoke extracts, which is expected to exert a lipid-lowering effect similar to berberine [18], with a high tolerability and safety profile [19].
In this context, our aim was to test the lipid-lowering effect of RYR and highly standardized artichoke extracts (ATC treatment) versus placebo and Armolipid Plus® in a double-blind, non-inferiority, randomized clinical study.
Material and methods
Trial design and participants
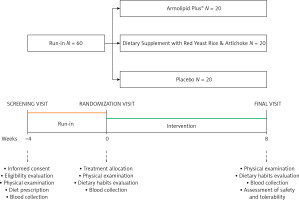
This was a three-arm, double-blind, non-inferiority, randomized, placebo-controlled, parallel-group clinical study that involved 60 healthy adult volunteers aged 18–70 years with a diagnosis of polygenic hypercholesterolemia and 115 mg/dl ≤ LDL-C ≤ 160 mg/dl. In order to enroll only healthy subjects, patients with obesity (body mass index (BMI) ≥ 30 kg/m2), uncontrolled hypertension (ambulatory systolic blood pressure ≥ 140 mm Hg and or diastolic blood pressure ≥ 90 mm Hg, either with or without pharmacological treatment), diabetes mellitus, a personal history of atherosclerosis-related cardiovascular diseases (ASCVD) (i.e. coronary artery disease, cerebrovascular disease, ultrasound diagnosed carotid atherosclerosis) or an estimated 10-year primary risk of ASCVD ≥ 10%, TG > 400 mg/dl, known current thyroid, gastrointestinal or active liver diseases, neuromuscular diseases or creatine kinase (CK) > 3 upper limit of norm (ULN), active cancer and any medical or surgical condition that could limit adherence to the full protocol were excluded from the study, as well as individuals already treated with lipid-lowering drugs, nutraceuticals, and any drugs that might have interfered with the investigated outcomes.
The study included a 4-week run-in period of diet standardization and an 8-week treatment period. For the entire study, enrolled volunteers were adhering to the general indications of a Mediterranean diet, avoiding an excessive intake of diary and red meat derived products and reducing dietary excesses, in order to maintain an overall balanced diet [20]. No specific suggestion to modify physical activity habits was made before or during the study. Before and after treatment, patients were evaluated for clinical status, and by the execution of a physical examination and laboratory analyses. All the assessments were carried out following standardized protocols. The study timeline is presented in detail in Figure 1.
The study fully complied with the ethical guidelines of the Declaration of Helsinki and with the International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Harmonized Tripartite Guideline for Good Clinical Practice (GCP), and its protocol was approved by the Ethical Committee of the University of Bologna. All patients signed written informed consent to participate.
Treatment
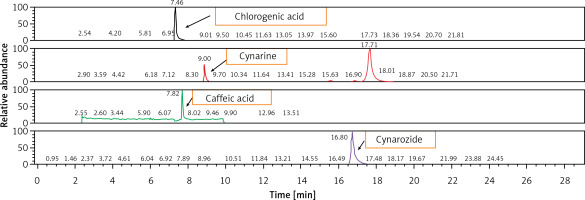
After a 4-week period of diet standardization, enrolled subjects were allocated to receive the active treatments (1 pill per day) containing low-dose monacolins from red yeast rice (2.8 mg per daily dose) associated with berberine or highly standardized artichoke extract, or placebo (Table I) in a 1 : 1 : 1 allocation ratio. The HPLC fingerprint of the tested artichoke extract is presented in Figure 2. Blocks were further stratified by sex and age. An alphabetical code was assigned to each lot code impressed on the dose box (one for each active treatment and for placebo). Investigators and the study staff, as well as all participants, were blinded to the group assignment. Codes were kept in a sealed envelope which was not opened until the end of the trial. Dose boxes were mixed and a blinded dose box was assigned to the patients.
Table I
Qualitative composition of the dietary supplements tested in the study
Participants’ compliance was assessed by counting the number of capsules returned at the time of the last visit. All the treatments were kindly provided by Meda Pharma S.p.A., Monza – MB, Italy.
Assessments
The patient’s history was evaluated with particular attention to ASCVD and other relevant diseases, dietary and smoking habit assessment, physical activity and ongoing pharmacological treatments. In particular, physical activity was assessed and monitored through the administration of a validated questionnaire based on the intensity and duration of physical activity section in a standard week [21].
Height and body weight were measured by standard procedures to the nearest 0.1 cm and 0.1 kg respectively, with subjects standing erect with eyes directed straight ahead, wearing light clothes, and with bare feet. Waist circumference (WC) was measured at the end of a normal expiration, in a horizontal plane at the midpoint between the inferior margin of the last rib and the superior iliac crest.
Blood pressure (BP) measurements were recorded in each patient supine and at rest, with a cuff of appropriate size applied at the right upper arm and by the use of a standard mercury sphygmomanometer (Erkameter 3000, ERKA, Bad Tolz, Germany; Korotkoff I and V). Following the latest European guidelines on BP measurement [22], BP measurements were always performed early in the morning, after the patient had rested for almost 10 min in a quiet room. The mean value of three BP readings – obtained at 1-minute intervals from each other – was considered as the variable of the study. Mean pulse pressure (PP) was calculated as the difference between systolic (SBP) and diastolic blood pressure (DBP) [23]. Enrolled volunteers were advised to avoid physical exercise, coffee (and other sources of caffeine) and smoking 2 h before the testing procedure.
Laboratory data
Hematochemical analyses were carried out on venous blood, withdrawn early in the morning from the basilica vein. At the time of sampling, subjects were fasted for at least 12 h. The biochemical variables investigated were: TC, high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), apolipoprotein B (Apo-B), apolipoprotein AI (Apo-AI), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), creatine kinase (CK), fasting plasma glucose (FPG), serum uric acid (SUA) and creatinine. All the laboratory analyses were carried out following a standardized method in a certified laboratory by trained personnel.
LDL-C was obtained by the Friedewald formula (LDL-C = TC – HDL-C – TG/5) [24]. The glomerular filtration rate (eGFR) was estimated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-epi) equation [25]. Lipid accumulation product (LAP) was calculated as (WC – 65) × TG (expressed in mmol/l) for men and (WC – 58) × TG (expressed in mmol/l) for women [26, 27]. The hepatic steatosis index (HSI) resulted from 8 × AST/ALT ratio + BMI (+2 for women) [28, 29]. Both LAP and HSI are validated indexes of non-alcoholic fatty liver disease and associated with cardiometabolic risk [30, 31].
Tolerability and safety assessment
Tolerability was assessed based on subjective reporting of any adverse events at the end of the study. Safety was assessed based on clinical and laboratory parameters at the end of the study.
Statistical analysis
Data were analyzed using intention to treat by means of SPSS Statistics version 21.0 for Windows. For the non-inferiority test, considering a mean baseline LDL-C level of 160 ±15 mg/dl, with a power of 0.80, an α error of 0.05 and non-inferiority delta of 5 mg/dl, a sample size of 18 subjects per active treatment group was calculated to be adequate. For the superiority test versus placebo, the sample size suggested to detect a mean LDL-C reduction of about 15 ±2%, with a power of 0.80 and an α error of 0.05, was 15 subjects per treatment group. As per protocol, we decided a priori to check the efficacy of treatments in subjects assuming at least 90% of the tested product doses foreseen by the trial design. Normally distributed baseline characteristics of the population were compared using Student’s t test (preceded by Levene’s test for equality of variances) and the χ2 test followed by Fisher’s exact test for categorical variables. Between-group differences were assessed by the analysis of variance (ANOVA) followed by Tukey’s post-hoc test. A linear regression analysis was carried out to detect a potential correlation between basal LDL-C level and percentage LDL change. All data were expressed as means and 95% confidence intervals. A two-tailed p level of < 0.05 was considered statistically significant for all tests.
Results
We consecutively enrolled 60 adult individuals (men = 28; women = 32), who were randomly assigned to the ATC group, to treatment with Armolipid Plus® or placebo. During the clinical study, no patient experienced any subjective or laboratory adverse events (dropout rate = 0%) and no patient was lost to follow-up. Thus, all the volunteers completed the study according to its design.
After the run-in period, no statistically significant differences were detected among the groups, which were well matched for all the considered variables at baseline, despite relatively large confidence intervals of some parameters such as TC and LDL-C (Table II). No statistically significant changes were recorded in the dietary habits (i.e. in total energy and macronutrient intake) of the enrolled individuals from randomization until the end of the study. Similarly, self-reported physical activity did not change for the participants over the course of the clinical trial.
Table II
Baseline main clinical and hematochemical characteristics of the subjects enrolled in the trial and randomized to the different treatments
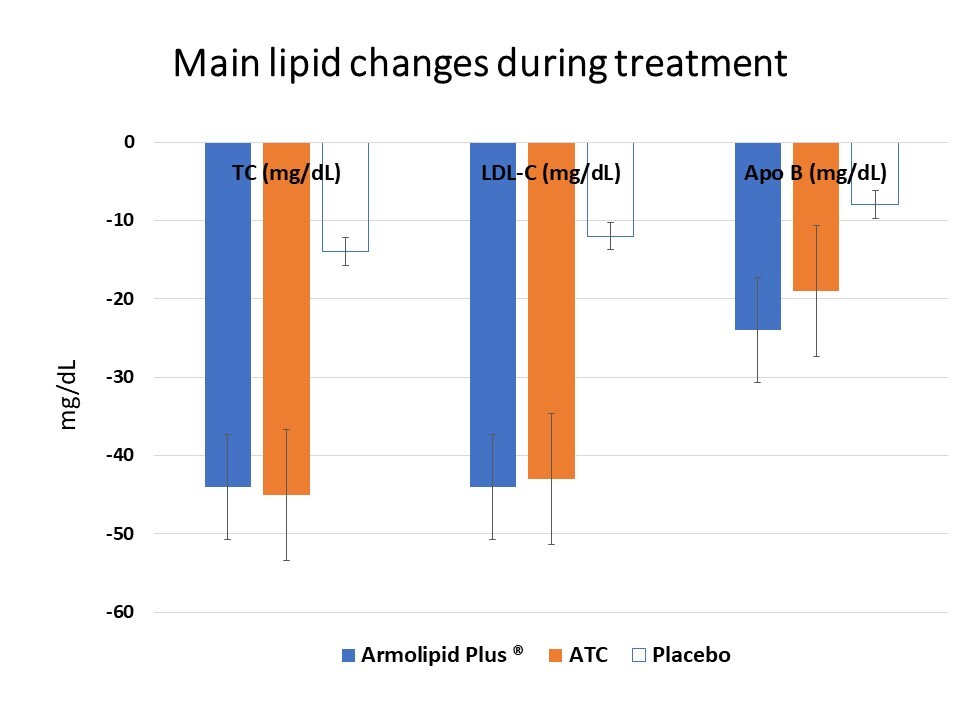
Regardless of the randomization group, there was no pre-post intervention change in SBP, DBP, PP, Apo-A, SUA, FPG, creatinine, eGFR, CK, GGT, AST, ALT and LAP (Table III). However, patients in all groups experienced significant improvements in baseline TC, LDL-C, Apo-B, TC/HDL-C ratio, LDL-C/HDL-C ratio and Apo-B/Apo-AI ratio (all p < 0.01), and the extents of variation depended on the administered treatment (ATC group: TC = –18.9%, LDL-C = –26.7%, ApoB = –19.6%, TC/HDL-C ratio = –18.8%, LDL-C/HDL-C ratio = –27.3%, Apo-B/Apo-AI ratio = –20%; Armolipid Plus® group: TC = –18.4%, LDL-C = –25.8%, ApoB = –23.2%, TC/HDL-C ratio = –26%, LDL-C/HDL-C ratio = –32.9%, Apo-B/Apo-AI ratio = –21.4%; placebo: TC = –6.2%, LDL-C = –8%, apoB = –8.4%, TC/HDL-C ratio = –8.9%, LDL-C/HDL-C ratio = –10%, Apo-B/Apo-AI ratio = –13.3%). Remarkably, LDL-C variations in actively treated individuals were statistically significant not only compared to baseline but also compared to placebo (Table III). LDL-C changes were significantly reduced by 12.4% and 12.8% versus placebo in ATC and Armolipid Plus® treated subjects. Moreover, subjects in ATC and Armolipid Plus® groups respectively attained significantly lower BMI values and improved baseline HDL-C (+8.7%) and TG (–17.5%) levels (Table III). Finally, baseline WC, AST/ALT ratio and HSI significantly decreased in both the ATC and Armolipid Plus® groups (Table III). Overall, ATC and Armolipid Plus® experienced similar significant improvements in anthropometric measures such as WC and BMI (p < 0.05) compared to the baseline, with a parallel improvement in HDL-C and TG, and, consequently, a decrease in adherence to the metabolic syndrome diagnostic criteria.
Table III
Changes in the main parameters evaluated at the end of the intervention period (after 8 weeks of placebo or active treatment)
| Parameter | Placebo | Dietary supplement with red yeast rice and artichoke | Armolipid Plus® | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean delta versus baseline | 95% confidence interval | P versus baseline | Mean delta versus baseline | 95% confidence interval | P versus baseline | Mean delta versus baseline | 95% confidence interval | P versus baseline | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Waist circumference [cm] | –0.35 | –0.79 | 0.09 | 0.110 | –0.50 | –0.97 | –0.03 | 0.038 | –0.74 | –1.36 | –0.12 | 0.022 |
| Body mass index [kg/m2] | –0.16 | –0.37 | 0.05 | 0.127 | –0.19 | –0.37 | –0.01 | 0.045 | –0.21 | –0.43 | 0.01 | 0.055 |
| Systolic BP [mm Hg] | 0.90 | –5.24 | 7.00 | 0.762 | 0 | –5.98 | 5.98 | 1.000 | –1.50 | –8.61 | 5.61 | 0.664 |
| Diastolic BP [mm Hg] | –0.20 | –4.47 | 4.07 | 0.923 | –2.10 | –7.02 | 2.28 | 0.383 | –2.05 | –6.40 | 2.30 | 0.336 |
| Pulse pressure [mm Hg] | 1.10 | –4.69 | 6.89 | 0.695 | 2.10 | –2.04 | 6.24 | 0.302 | 0.55 | –5.23 | 6.33 | 0.844 |
| Total cholesterol [mg/dl] | –13.75 | –18.00 | –9.50 | < 0.001 | –44.65 | –52.81 | –36.49 | <0.001 | –44.25 | –55.97 | –32.53 | < 0.001 |
| HDL cholesterol [mg/dl] | 0.40 | –2.65 | 3.45 | 0.787 | –0.25 | –2.91 | 2.41 | 0.846 | 3.95 | 0.26 | 7.64 | 0.037 |
| Triglycerides [mg/dl] | –11.70 | –30.87 | 7.47 | 0.217 | –4.85 | –31.45 | 21.75 | 0.707 | –20.25 | –40.37 | –0.13 | 0.049 |
| LDL cholesterol [mg/dl] | –11.81 | –16.23 | –7.39 | < 0.001 | –43.43§ | –50.83 | –36.03 | <0.001 | –44.15§ | –55.66 | –32.64 | < 0.001 |
| Total cholesterol/HDL cholesterol ratio | –0.41 | –0.71 | –0.11 | 0.010 | –0.92 | –1.22 | –0.62 | <0.001 | –1.38 | –1.89 | –0.86 | < 0.001 |
| LDL cholesterol/HDL cholesterol ratio | –0.32 | –0.57 | –0.07 | 0.014 | –0.89 | –1.16 | –0.63 | <0.001 | –1.25 | –1.72 | –0.78 | < 0.001 |
| Apo-A1 [mg/dl] | 7.20 | –2.36 | 16.76 | 0.131 | 5.95 | –3.73 | 15.63 | 0.214 | –3.10 | –12.03 | 5.83 | 0.476 |
| Apo-B [mg/dl] | –7.65 | –12.16 | –3.14 | 0.002 | –18.70 | –24.97 | –12.43 | <0.001 | –24.35 | –32.86 | –15.85 | < 0.001 |
| Apo-B/Apo-A1 ratio | –0.08 | –0.12 | –0.03 | 0.002 | –0.14 | –0.20 | –0.07 | <0.001 | –0.15 | –0.21 | –0.09 | < 0.001 |
| HDL cholesterol/Apo-A1 ratio | –0.01 | –0.03 | 0.01 | 0.392 | –0.02 | –0.04 | –0.01 | 0.021 | –0.01 | –0.03 | 0.02 | 0.741 |
| LDL cholesterol/Apo-B ratio | 0 | –0.09 | 0.09 | 0.961 | –0.15 | –0.25 | –0.04 | 0.007 | –0.08 | –0.24 | 0.09 | 0.347 |
| Serum uric acid [mg/dl] | 0.04 | –0.22 | 0.29 | 0.775 | –0.23 | –0.50 | 0.05 | 0.100 | –0.17 | –0.47 | 0.14 | 0.277 |
| Glucose [mg/dl] | –1.00 | –3.28 | 1.28 | 0.371 | –1.60 | –5.63 | 2.43 | 0.416 | –0.65 | –0.41 | –3.95 | 0.685 |
| Creatinine [mg/dl] | –0.02 | –0.07 | 0.03 | 0.408 | –0.01 | –0.04 | 0.05 | 0.834 | –0.02 | –0.07 | 0.04 | 0.545 |
| Estimated glomerular filtration rate [ml/min/1.73 m2] | 1.15 | –4.14 | 6.44 | 0.654 | –1.35 | –5.82 | 3.12 | 0.535 | 1.05 | –3.74 | 5.84 | 0.651 |
| Creatinine kinase [U/l] | 29.60 | –4.32 | 63.52 | 0.084 | –14.20 | –38.68 | 10.28 | 0.240 | –5.20 | –35.51 | 25.11 | 0.724 |
| γ-glutamyltransferase [U/l] | 1.00 | –1.93 | 3.93 | 0.484 | –1.65 | –7.09 | 3.79 | 0.533 | 4.75 | –3.61 | 13.11 | 0.249 |
| Aspartate aminotransferase [U/l] | 1.10 | –0.43 | 2.63 | 0.148 | 0.50 | –3.60 | 4.60 | 0.801 | 3.25 | –0.28 | 6.78 | 0.069 |
| Alanine aminotransferase [U/l] | 1.10 | –1.78 | 3.98 | 0.433 | 1.90 | –5.73 | 9.53 | 0.608 | 2.95 | –1.64 | 7.54 | 0.195 |
| Aspartate aminotransferase/alanine aminotransferase ratio | –0.03 | –0.09 | 0.04 | 0.424 | –0.08 | –0.14 | –0.02 | 0.013 | –0.11 | –0.20 | –0.03 | 0.014 |
| Lipid accumulation product | –4.37 | –10.57 | 1.84 | 0.158 | –0.97 | –9.29 | 7.35 | 0.810 | 3.32 | –1.75 | 8.40 | 0.187 |
| Hepatic steatosis index | –0.37 | –0.90 | 0.17 | 0.171 | –0.83 | –1.36 | –0.31 | 0.003 | –1.12 | –1.82 | –0.41 | 0.004 |
Discussion
In this double-blind, placebo-controlled, randomized clinical trial, we found that two lipid-lowering nutraceutical approaches exerted similar LDL-lowering effects (–26% vs. baseline, –12% vs. placebo) after 8 weeks of treatment on top of a standard Mediterranean diet. Both tested nutraceuticals also had similar reducing effects on TC and Apo-B plasma level, but also a small but significant improving effect on HSI, a validated marker of liver steatosis [30, 31]. Interestingly, individuals in the ATC group attained significantly lower BMI values (–2.1%), while individuals treated with Armolipid Plus® had improvements in baseline HDL-C (+8.7%) and TG (+17.5%) HDL-C and TG levels. The small, but statistically significant, improvement observed in the control group as regards TC and TC related parameters supports the assumption that they complied with dietary advice.
Even though the metabolic effect of low-dose RYR alone and in association with berberine has already been adequately investigated and quantified in a number of meta-analyses of RCTs [32–34], no placebo-controlled study previously compared the effect of Armolipid Plus® versus other lipid-lowering nutraceutical compounds.
Depending on the mechanism of action, nutraceuticals with a detectable lipid-lowering effect can be classified as natural inhibitors of intestinal cholesterol absorption, inhibitors of hepatic cholesterol synthesis, and enhancers of LDL-C excretion [29]. However, the lipid-lowering effect of most nutraceuticals occurs through multiple mechanisms, and the interaction between RYR and other natural products with different mechanisms of action – such as berberine, policosanols and chlorogenic acid from artichoke leaves – has additive or synergistic lipid-lowering effects [18].
In this three-arm, double-blind, placebo-controlled clinical study, individuals randomized to either RYR and artichoke extracts or Armolipid Plus® experienced a significant decrease in LDL-C compared to baseline and placebo. Moreover, dietary supplementation with Armolipid Plus® exerted significant reductions in TG, which have to be attributed to the berberine-induced activation of AMP-activate protein kinase (AMPK) pathway, which determines an increase in fatty acid oxidation and a reduction in the expression of lipogenic genes [35, 36]. On the other hand, chlorogenic acid from artichoke leaves has been suggested to reduce cholesterolemia by two different mechanisms of action: (i) interacting in the liver with the regulation pathways of the acetyl-CoA C-acetyltransferase (ACAT) and the sterol regulatory element binding proteins (SREBPs) – which are paradoxically elevated in patients with non-alcoholic steatohepatitis (NASH) – and (ii) by interaction of lutein with the enzyme HMG-CoA reductase [37].
Overall, the present findings are in line with previous observations from another RCT [38], which however did not compare dietary supplementation with RYR and artichoke extracts with the most widely used Armolipid Plus® [39]. Additional RCTs investigated the lipid-lowering effect of artichoke extract alone and highlighted a tendency to improve plasma lipids, which was not however comparable to the results that can be obtained in clinical practice after RYR monacolin K monotherapy [40]. In our study, treatment with RYR and artichoke extracts was able to induce a significant reduction in LDL-C plasma level and related parameters. Moreover, both the tested dietary supplements were associated with a significant improvement in baseline AST/ALT ratio and HSI. Based on pre-clinical and clinical investigations, artichoke leaf extract owes its antioxidant properties to the main constituents, namely caffeic acid, volatile sesquiterpene and flavonoids, including the glycosides luteolin-7β-rutinoside (scolymoside), luteolin-7β-D-glucoside and luteolin-4β-D-glucoside and mono- and dicaffeoylquinic acid (cynarin and chlorogenic acid) [41, 42]. Our findings substantially confirm the hepatoprotective effect of berberine and artichoke leaf extract and, of consequence, provide data supporting their use in individuals with non-alcoholic fatty liver disease (NAFLD) or NASH and dyslipidemia, and in statin-intolerant patients with high transaminases [37, 43]. Overall, the tested products have a similar impact on a relatively large number of metabolic parameters; hence artichoke extract could replace berberine when berberine is not usable for local administrative reasons.
Remarkably, no side effect was recorded during the study, supporting the good tolerability and safety profile of the new tested supplement combination (ATC) in the short-medium term, whereas the long-term side effects of Armolipid Plus® have already been demonstrated [44, 45].
Even if longer studies are needed to confirm the observed results, the study duration is sufficient to detect the lipid-lowering efficacy of the tested product. Of course, we acknowledge that our study has some limitations, namely the short study duration and the small patient sample size, even though the study was powered for the primary outcome measure. Our observations cannot be generalized to patients with obesity, since BMI ≥ 30 kg/m2 was an exclusion criterion for the study. Moreover, as above stated, the study was relatively short, so we do not know whether the observed efficacy and safety data could be confirmed in the long term [46]. Finally, further studies on vascular outcomes – such as arterial stiffness or endothelial function – are required to evaluate whether dietary supplementation with RYR and artichoke extracts are able to exert any vascular effect.
In conclusion, in the present clinical study, dietary supplementation with RYR and artichoke extracts was shown to be as effective as Armolipid Plus® in improving a number of CV risk factors in the short term, suggesting that this nutraceutical combination may be advantageously and safely used in patients with hyperlipidemia when berberine is not usable for local administrative reasons. Available clinical data are preliminary, so further confirmation is required. However, our observations are relevant and consistent with previous literature, and certainly deserve to be further investigated in controlled clinical trials with longer-term follow-up.