Introduction
Acute mesenteric ischemia (AMI) is a syndrome that can lead to acute abdominal pain. It has a poor prognosis despite recent developments in its diagnosis and treatment. Mortality rates in AMI still remain at 50% to 69% [1]. Although AMI is only seen in 0.1% of hospital admissions and 1% of emergency department visits, the incidence is increasing due to an aging general population with increasing prevalence of comorbidities [1]. Survival exceeds 90% with early diagnosis. It is important to restore blood flow to the intestine following the onset of ischemia within the first 6 h to improve prognosis especially in the case of ischemia related to embolism [2]. New and facile diagnostic tools such as serum and peritoneal indicators can assist with early diagnosis to decrease mortality. Various other indicators such as levels of lactate dehydrogenase, D-dimer, potassium, pH, ischemia-modified albumin, urine, and fatty acid-binding proteins have also been described [3–11]. Reports that D-dimer, potassium, and pH can guide the early diagnosis of AMI have gained recent attention [4, 11–13].
D-dimer is a fibrin degradation product stabilized by factor XIII. It is produced as part of the normal wound healing process and blood clot formation [14]. First used in the 1990s, D-dimers levels are now used when there is suspicion of a thrombotic disorder. It is a valuable indicator of the presence of unwanted thrombotic events such as when clotting forms pathologically or as result of disease such as underlying disseminated intravascular coagulation (DIC), deep vein thrombosis (DVT), pulmonary embolism, coronary heart disease, or venous thrombosis. Though a negative test result essentially excludes thrombosis, a positive result does not conclusively indicate thrombosis. Therefore, the test is primarily used to eliminate thromboembolic disease in cases where the probability is low. The sensitivity and specificity of the D-dimer test in the diagnosis of thrombotic disease are 93% to 95% and 50% respectively. False positive results can arise due to liver disease, high rheumatoid factor, inflammation, malignancy, trauma, pregnancy, recent surgery, or advanced age. False negative results may occur if the blood sample is taken immediately after thrombus formation or if the test is delayed for a few days [15].
This rat study used an induced AMI model to evaluate levels of potassium, pH, and D-dimer in the blood as well as potassium and pH in peritoneal lavage fluid to assess their efficacy in the early diagnosis of AMI.
Material and methods
This study was conducted at the Istanbul University Center of Experimental Medicine with approval from the Istanbul University animal testing ethics committee (approval protocol no: 2015/63/06.04.2015). All experiments were performed in accordance with the National Institutes of Health guidelines for the care and handling of animals (Bethesda, MD, USA). An exhaustive review of the written literature to detect prior related research was performed via Science Direct and PubMed; all linked data were collected and recorded.
Male albino Wistar rats (n = 24; 250 to 350 g) were divided into four groups of six rats as groups A, B, C, and D. All rats were kept in metallic cages at room temperature for 22 ±2°C with a relative humidity of 40–65% with a 12-hour light/dark cycle throughout the experimental period. They were fed a standard pellet feed and given tap water; they were not given any food one day prior to the procedure. Rats were housed in 4 separate cages according to groups.
The anesthesia was composed of ketamine hydrochloride 50 mg/ml (Ketalar; Pfizer, Inc., NY, NY, USA) and xylazine hydrochloride 20 mg/ml (Alfasan International B.V., Woerden, The Netherlands) at 2 : 1. This was administered intraperitoneally at 0.1 ml/100 g. After administering anesthesia and applying antiseptic, a 4-cm median laparotomy was performed. Groups A and B were the control groups for groups C and D respectively. Groups A and B had no mesenteric procedure. Groups C and D had a superior mesenteric artery (SMA) that was meticulously isolated and ligated with 1-0 silk. A single-lumen 14-F venous catheter was passed through the anterior abdominal wall, positioned inside the peritoneum, and fixed to the abdominal skin. The abdomen was closed with a 4-0 monofilament nylon suture, which remained closed until the end of the experiment. Acetaminophen (50 mg/kg s.c.) was administered analgesically to all rats during the observation time. The rats were then allowed to recover from the anesthesia. Peritoneal lavage was performed on each rat with 5 ml of warm physiological serum via a secured venous catheter. The procedure was conducted 60 min after closure of the abdomen in groups A and C and 120 min after closure for rats in groups B and D.
A 2 ml sample of lavage fluid was withdrawn for the workup, and 6 cc of blood was simultaneously taken from the cardiac cavity of all rats. The potassium, pH, and D-dimer were measured in blood, and potassium and pH were measured in the peritoneal lavage fluid. Blood samples taken for potassium and pH measurements were collected in blood gas syringes prepared via 2 cc of lithium-heparin. Coagulation tubes were 2-ml sodium-citrate for the D-dimer measurements. Peritoneal lavage fluid samples were collected in 2-cc heparinized blood gas syringes for potassium and pH analysis. All samples were preserved on ice in storage containers and transported to the laboratory within 1 h from collection. The blood samples taken for potassium and pH were analyzed without being centrifuged. The D-dimer samples were centrifuged at 4000 rpm for 10 min and then studied using an automated plasma analyzer.
The rats were immediately sacrificed after receiving the blood sample. The sutures of the abdomen were opened and the entire intestinal tract was removed and fixed in 10% neutral formaldehyde. Tissue samples were stained with hematoxylin-eosin in 4-μm sections. Two researchers examined every slide and blindly graded the intestinal ischemia damage using a 6-tiered scale defined by Ji et al. [16].
Statistical analysis
Statistical results were obtained by comparing groups A with C, groups B with D and groups A with B. Statistical analyses used SPSS software (version 15; IBM Corp., Armonk, NY, USA). The Mann-Whitney test, a nonparametric test, was used to compare two independent groups. The intestinal ischemia levels were compared between groups using a χ2 test. Results were expressed within a 95% confidence interval. Significance was defined as p < 0.05.
Results
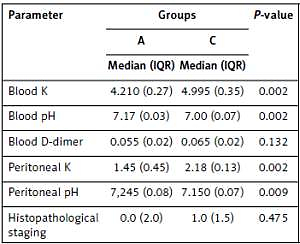
Median (IQR) and p values are presented in Tables I and II as a result of comparisons of groups A and C and groups B and D, respectively. Groups C and D (mesenteric ischemia model) had statistically significant differences in potassium and pH in blood and peritoneal lavage fluid versus groups A and B (p < 0.05). There was no significant difference between groups C and A in terms of D-dimer in blood or histopathological staging results. However, there was a significant difference between groups D and B in terms of D-dimer in blood (p = 0.002) or histopathological staging (p = 0.019) results.
Table I
Median (IQR) and p-values compared to groups A and C
Table II
Median (IQR) and p-values compared to groups B and D
Strict suture closure and involuntary intestinal injury may have affected the results. Thus, groups A and B were compared in terms of biochemical parameters and histopathologic stage; no significant differences were observed. This result showed that the surgical procedure did not lead to potential confounders such as inflammation and increased intra-abdominal pressure in our study. There was no mortality during the experiment.
Discussion
The AMI occurs as result of severe reduction or cessation of oxygen flow to the intestines. It can develop after shock or from occlusion of mesenteric arterial flow or venous drainage. The most common pathophysiological mechanisms are superior mesenteric artery (SMA) embolism, SMA thrombosis, and non-occlusive mesenteric ischemia [1].
The rate of AMI in patients admitted to the emergency service is 1 : 1000 [1]. Difficulties and delays in diagnosis before necrosis can lead to a high rate of AMI mortality. The mortality rate in patients diagnosed more than 24 h after AMI is 20% higher [17]. In a series with 21 cases of AMI, intestinal vigor was reported at 100% in patients diagnosed before 12 h had passed from the onset of symptoms. This became 56% of patients diagnosed between 12 and 24 h after onset, and 18% in patients diagnosed after 24 h had passed [1]. Extensive intestinal and even colon resections are required in patients with delayed diagnosis. Short bowel syndrome manifests in surviving patients when there is not enough intestine for adequate nutrition.
Numerous studies have reported different determinants that could be useful in the early diagnosis of AMI. However, it is obvious that these determinants are not used in daily practice because there is no consensus on the matter. It would be advantageous to confirm a determinant that could be used in daily practice for early diagnosis of cases with suspicion of AMI. The novelty of this study is to emphasize the importance of determinants that we can easily use in daily practice in the early diagnosis of AMI. Similar results have been obtained in two experimental studies in which pH and potassium levels were studied in the blood and peritoneal lavage fluid. These studies showed an early decrease in the pH of peritoneal lavage fluid and an increase in potassium. Consequently, experimental ischemia reports have shown that pH and potassium levels in peritoneal lavage fluid could be useful for early diagnosis when the blood pH has not yet increased [11].
Metabolic acidosis is generally accepted as a late symptom of AMI and is another determinant that can contribute to early diagnosis. For instance, an incidental finding of elevated D-dimer levels in patients with SMA embolism can arouse interest. In 2000, a Swedish group measured D-dimer levels on the basis of this finding to ascertain whether a wider prospective study would be worth conducting in patients with suspected AMI. They reported that high levels of D-dimer indicated intestinal ischemia [12]. A study of 50 patients by Yang et al. reported that D-dimer levels are an efficient, early, and specific serum determinant that could affect the clinical course; it can indicate acute superior mesenteric venous thrombosis [18].
Recently, Coşkun et al. studied increased D-dimer and neopterin levels in the blood of rabbits with induced AMI including their utility in early diagnosis of AMI [19]. They reported substantial statistically significant differences in both D-dimer and neopterin. In 2 other experimental studies on rats, plasma D-dimer levels measured after SMA occlusion were significantly higher in ischemic groups. It was concluded that D-dimer measurements could be useful in the early diagnosis of AMI [20, 21]. Another study concluded that biphasic computed tomography (CT) and mesenteric CT angiography have similar utility as D-dimer for AMI diagnosis. Experts have reported that CT and CT angiography cannot be performed in cases with renal failure and contrast hypersensitivity, but D-dimer tests are broadly and easily applicable with high sensitivity and specificity [13].
Here, time-dependent changes in the levels of potassium and pH in the peritoneal fluid as well as levels of potassium, pH, and D-dimer in blood were demonstrated as a potential marker in an AMI experimental rat model. Correlations between the levels of these markers and histopathological intestinal damage were analyzed. The levels of potassium, pH, and D-dimer were compared for the early diagnosis of AMI. These markers were chosen because they were common in everyday practice. Both potassium and pH showed a significant difference in a relatively short time (~1 h); D-dimer levels in blood showed a significant difference by the second hour, but this is quite sufficient for early diagnosis. However, D-dimer levels were better correlated with ischemic damage. All of these markers were identified as useful biomarkers for the early detection of AMI because they reached significant levels within 6 h.
There were limitations to our study. Other factors can increase D-dimer levels in humans, and using them for AMI diagnosis is controversial. Moreover, the levels of AMI determinants in rats and humans may be different. Diagnostic percutaneous peritoneal lavage in human patients can be considered as a difficult procedure. However, percutaneous or surgical peritoneal lavage may be performed for early diagnosis of human diseases by encouraging positive results in animal experiments. In some recent studies, percutaneous peritoneal lavage sampling has been used for rapid staging in patients with stomach and pancreatic cancer [22, 23]. The routine mesenteric ischemia model with ligation was used here, and the markers may be expressed differently in the model than the clinic. This question could be resolved if different ischemia models were constructed in larger groups. Chronic diseases may change the levels of the determinants over time. Here, a cut-off value for the D-dimer level could not be determined because the number of rats was too low for statistical evaluation. This value would not apply to the clinic regardless.
In conclusion, although many experimental studies have been designed to assist in early diagnosis of AMI, the results of these studies are not usually translated to clinical practice. Therefore, determinants already used frequently in clinical practice might be more useful. We found that the levels of potassium, pH, and D-dimer could be useful in daily practice for the early diagnosis of AMI.