Introduction
Diabetes mellitus, a chronic metabolic disease, is believed to be the most common hormonal disease in the world [1]. It is estimated that diabetes can cause chronic kidney disease and end-stage renal disease [2]. It has also been increasingly assumed that the renal tubulointerstitium takes part in the pathogenesis of diabetic nephropathy with extended exposure to a variety of metabolic and haemodynamic injuring factors associated with sustained hyperglycaemia as a contributing factor [3].
Diabetes mellitus (type 1 diabetes, type 2 diabetes, gestational diabetes) is a frequent medical complication in pregnancy [4]. Not only is the mother adversely affected but also the child, causing early perinatal complications and long-term consequences. There is vast body of evidence that, regardless of diabetes and hyperglycaemia during pregnancy, the risk of adverse events increases [5, 6].
Neutrophil gelatinase-associated lipocalin (NGAL) produced in epithelial cells and neutrophils in most tissues is a small (25-kD) protein. It is a marker of renal tubular damage [7]. Also, kidney injury molecule-1 (KIM-1), a transmembrane protein present only in the proximal tubules of injured human kidneys, can be considered as a marker of acute proximal tubular kidney damage [8].
Until now, no study concerning the impact of diabetes during pregnancy on the functions of newborns’ kidneys has been conducted using early biomarkers of kidney damage.
The purpose of the study was to test the differences in concentrations of urine NGAL and KIM-1 between newborns exposed to maternal diabetes during pregnancy and newborns from normal pregnancies.
Material and methods
Patient recruitment and sample collection
In the prospective study 138 neonates were included. They were born on time at the Department of Perinatology and hospitalised at the Department of Neonatology and Intensive Care, Medical University of Bialystok, Poland at a rooming-in ward. Fifty newborns were from pregnancies complicated by diabetes mellitus (11 by type 1 diabetes, 17 by gestational diabetes requiring insulin therapy, 22 by gestational diabetes requiring a diet therapy, who were diagnosed using WHO 2013 criteria [9]).
Eighty-eight healthy newborns resulted from normal pregnancies with no prenatal and perinatal complications. Newborns included in the study met the following criteria: normal physical examination and normal prenatal ultrasound examination of the kidney. Neonates with a history of any congenital anomaly, Apgar score lower than 8 in the first minute of birth, respiratory disorders, heart illnesses, intrauterine infections, and higher markers of inflammation were not included in the analysis.
The Local Bioethics Committee of the Medical University of Bialystok approved the protocol of the study. We conducted the procedures according to the Helsinki Declaration. We received written informed consent from parents of all the newborns before the study.
Samples of urine using single-use sterile bags (UNIDEM, London) were taken from the study group. We collected urine and blood samples on the first or second day of life. The blood samples were drawn during routine practice in the unit, using S-Monovette 1.2 ml, Clotting Activator/Serum test tubes (Sarstedt AG & Co, Germany). The samples of serum and urine after centrifugation were kept in the fridge (4°C) for a maximum of 2 h and then frozen at –80°C. Repeated freeze-thaw cycles were avoided.
Determination of urine NGAL
We measured the levels of urine NGAL using an ELISA kit (R&D Systems, Minneapolis, MN, USA & Canada), which is commercially available. We measured the levels according to the manufacturer’s instructions, with results in nanograms per millilitre. The detection limit was < 0.1 ng/ml. The mean intra- and inter-assay coefficients of variation (CV) for urine NGAL were 3.4 and 11.4%, respectively.
Determination of urine KIM-1
We determined the urine concentrations of KIM-1 using an ELISA kit (R&D Systems, Minneapolis, MN, USA & Canada), which is commercially available. We measured the concentrations according to the manufacturer’s instructions, with results in nanograms per millilitre (ng/ml). The limit of detection of KIM-1 levels was 0.07 ng/ml.
We normalised the urine concentrations of NGAL and KIM-1 for urine concentration of creatinine measured by Jaffé’s method (uNGAL-1/cr., uKIM-1/cr.) to account for a potential confounding effect of urine dilution. It was expressed in nanograms per milligram of creatinine (ng/mg cr.). The level of serum creatinine was determined by Jaffe’s method and shown in milligrams per decilitre. We calculated the estimated glomerular filtration rate (GFR) according to Schwartz formula (eGFR = 0.45 × L/Scr., where L is length in centimetres, and Scr. is serum creatinine in milligrams per decilitre).
Statistical analysis
We analysed the results using Statistica 12.0 package (StatSoft, USA). We showed discrete variables as counts (percentage), whereas continuous variables as median and quartiles (Q1–Q3). To determine the normal distribution, we used Shapiro-Wilk test, and if the data were not normally distributed, we used Mann-Whitney U-test for intergroup comparisons of continuous variables.
We compared the distributions of discrete variables with the χ2 test. Spearman’s coefficients of rank correlation measured the direction and the power of association between the concentrations of KIM-1 and other variables. We analysed the results of all the tests significant at p < 0.05.
Results
In total, 138 full-term newborns were included in the study. Fifty neonates (33 boys, 17 girls) were exposed to maternal diabetes during pregnancy (D). Eighty-eight healthy newborns (44 boys, 44 girls) –the reference group (R) – were born from normal pregnancies with no prenatal and perinatal complications. Both groups were sex-matched (p > 0.05). We also divided the examined group (D) into two subgroups: babies born from diabetic mothers treated only with a diet (D1) and with insulin therapy (D2). There were no complications in the pregnancies of mothers with diabetes. Forty mothers had glycated haemoglobin (HbA1c) < 6.0% without hypoglycaemia. The rest of them (mothers with gestational diabetes treated with a diet) reported that they had regularly measured blood glucose concentrations during the day and they were normal (fasting blood glucose levels < 5.3 mmol/l (95 mg/dl), 2 h after a meal < 6.7 mmol/l (120 mg/dl)).
The serum glucose concentrations in all newborns were normal.
In Table I we present the patients’ demographics, median values, and interquartile range (IQR) of measured parameters.
Table I
Characteristics of the study population – newborns from diabetic (D) and normal pregnancies (R)
Newborns from normal and those from diabetic pregnancies were born on time; however, the gestational age of neonates in group D was lower (p < 0.01). There were no significant differences between the parameters of physical development (birth weight, length, head and chest circumference). Significantly higher concentrations of NGAL and NGAL/cr. in urine were found in newborns from diabetic pregnancies comparing to the reference group (R) (p < 0.02; p < 0.01, respectively). Also, urine KIM-1 was higher in group D compared to the reference group (R), but the differences did not differ considerably. No significant differences in the concentration of creatinine and the estimated GFR in serum were found.
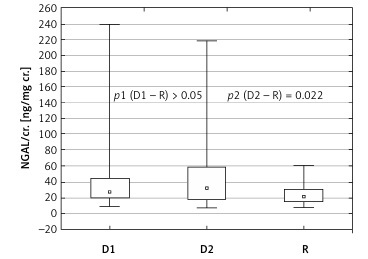
In Table II we compared measured parameters in subgroups on a diet (D1) and on insulin therapy (D2) with the reference group (R). Comparing the parameters between group D1 and R we only found that the gestational age in group D1 was lower than in group R (p < 0.05). No statistically relevant differences were observed in the concentrations of NGAL and KIM-1 between groups D1 and R. However, when comparing the subgroup on insulin therapy (D2) with group R, more statistical discrepancies were found between the groups than when the whole diabetic group (D) was compared with the reference group (R). In subgroup D2 the gestational age was significantly lower (p < 0.01). There was considerably higher concentration of NGAL, and also NGAL/cr. in the urine was significantly higher, in group D2 compared to group R (p < 0.02 and p < 0.03, respectively) (Figure 1).
Table II
Comparison of examined parameters in subgroups on diet (D1) and on insulin therapy (D2) with the reference group (R)
Figure 1
Urine NGAL/cr. depending on type of diabetes treatment
uNGAL – urine neutrophil gelatinase-associated lipocalin, cr. – creatinine, D1 – subgroup on diet therapy, D2 – subgroup on insulin therapy, R – reference group.
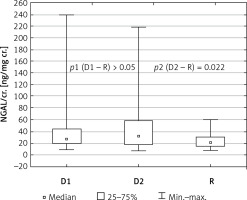
Statistically relevant differences were also found in the KIM/cr. ratio between these two groups. The median concentrations of KIM-1 were higher in group D2 compared to the reference group R, but these differences were not statistically significant.
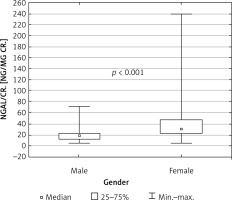
Because the groups were sex-matched, the association between female gender and higher concentrations of NGAL and also NGAL/cr. in the urine was found both in the whole group of neonates taking part in the study (p < 0.001) and in the group of neonates from diabetic pregnancies (p < 0.01) (Figure 2). The concentration of KIM-1 did not depend on sex.
Figure 2
Urine NGAL/cr. depending on gender in the whole study population – newborns from diabetic (D) and normal pregnancies (R)
uNGAL – urine neutrophil gelatinase-associated lipocalin, cr. – creatinine.
There were negative correlations between the urine NGAL/cr. and the week of pregnancy (R = –0.196, p = 0.02), birth weight (R = –0.381, p < 0.001), head circumference (R = –0.333, p < 0.001), and chest circumference (R = –0.330, p < 0.001). These correlations were not found in KIM-1/cr.
Newborns from normal and from diabetic pregnancies were born on time; however, the gestational age of newborns from group D was lower (p < 0.01). Newborns from pregnancies complicated by diabetes were born in our hospital a little earlier than normal pregnancies. After observation, we came to the conclusion that only urine NGAL/cr. correlated negatively with the week of pregnancy.
We used the factors that were found to have had a significant correlation with the urine NGAL/cr. ratio as explanatory variables to create multiple regression models. Some correlated variables were removed to reduce the impact of multicollinearity. In this model the remaining three parameters (gestational age, birth weight, male gender) comprised over 22% of the variations in the NGAL/cr. ratio level (R = 0.4754, R2 = 0.2261, p < 0.01).
Discussion
Nowadays, diabetes is more often regarded as the main cause of chronic renal failure, with many patients progressing to end-stage renal illness needing dialysis or transplantation [10]. The damage affects not only the glomerulus but also the renal tubules [11]. Histological studies show that proximal tubular basement membrane width is already thickened in normoalbuminuric diabetic patients compared to healthy controls [12]. Fioretto et al. observed that in microalbuminuric diabetic patients with similar GFR only 29% of patients had typical histological glomerular features of diabetic nephropathy, whereas 42% had severe tubulointerstitial lesions disproportional to mild glomerular involvement [13].
Not only are the early perinatal, diabetes-caused complications further complications of pregnancy, but they also have long-term consequences. The study by Nelson et al. provided evidence that maternal hyperglycaemia may lead to adult disease in offspring [14]. They concluded that the risk of elevated urine albumin excretion in diabetic Pima Indians is increased by exposure to a diabetic intrauterine environment [14]. Yan et al. observed early renal dysfunction of male newborns born by mothers with diabetes and moderate hyperglycaemia [15]. Also, in the urine of newborns born from diabetic mothers the presence of N-acetyl-b-D-glucosaminidase, an enzyme which reflects the degree of proximal tuber necrosis, was affected [15].
Patients with diabetes have elevated concentrations of urine NGAL and KIM-1, even if albuminuria is not observed [16–18]. The values of urine NGAL and KIM-1 in newborns born from pregnancies complicated by diabetes mellitus have been determined for the first time in this prospective study. Babies born by mothers with diabetes are exposed to permanent or periodic (in the case of balanced diabetes) hyperglycaemia. In the study on foetuses with exposure to maternal diabetes during pregnancy, Taricco et al. made a similar observation. These babies had higher concentrations of umbilical glucose, even if maternal glucose levels were normal, as well as the reduction in oxygen saturation and oxygen content with higher lactate concentration. It showed altered foetal metabolism [19]. Hyperglycaemia inducing the production of reactive oxygen species, leading to the accumulation of advanced glycosylated end-products and activation of protein kinase C, can in turn lead to tissue injury not only to the glomerulus, in particular podocytes, but also to the tubulointerstitium [20–22]. There are structural changes in the proximal tubules, such as tubular atrophy, interstitial fibrosis, and peritubular capillary rarefaction [19].
Unfortunately, there are no data on the levels of NGAL and KIM-1 in babies from pregnancies complicated by diabetes in the literature. Higher excretion of urine NGAL was, however, found in early-stage diabetes, e.g. type 1 in children [17, 23]. Taking into consideration the fact that urine N-acetyl-b-D-glucosaminidase is a marker of renal tubular damage, the observation made by Yan et al., who found an increase in excretion of this enzyme in the offspring of diabetic rats, may prove the importance of looking for markers of the coils’ damage in the group of newborns of mothers with diabetes [15]. In the study, we observed that the median urine NGAL and urine NGAL/cr. levels were significantly higher in newborns born by mothers with diabetes (p < 0.02; p < 0.01), and that the difference of urine NGAL concentration was even more significant between group D2 and the reference group (p = 0.01) and similar for NGAL/cr. (p < 0.02). We observed higher excretion of urine only in the group of babies from pregnancies complicated by diabetes treated with insulin compared to the reference group. It was probably associated with a higher risk of hyperglycaemia in type 1 diabetes compared to gestational diabetes. Analysing the results, we assumed at NGAL is a more sensitive and early marker of tubular damage than KIM-1. Other authors also found such results. The studies in newborns with AKI confirmed that NGAL was an important early biomarker of AKI, but data on KIM-1 were not conclusive [24–29]. Some authors indicated its statistically significant role as an early AKI biomarker [25], while others did not [26, 27]. In some publications the level of NGAL elevated rapidly after the activation of a damaging factor, whereas the increase in KIM-1 concentration was associated with the occurrence of proteinuria [28, 29].
It should also be mentioned that the level of NGAL depends on gender and is much lower in boys, which was proved in studies involving both children and adults [30, 31]. During our study, we found that urine NGAL concentration depended on sex and was higher in female gender both in the D and R groups. These differences were not found in KIM-1 concentrations. Multifactorial regression also confirmed our observation, in which we showed that gender had the biggest impact on the value of NGAL/cr.
It is also worth emphasising the fact that babies qualified for the study were born from pregnancies complicated by diabetes but properly compensated. Newborns were born on time, and no features of diabetic fetopathy were observed. Therefore, it should be assumed that changes in the kidney tubules might not be very advanced. We should note that in children from group D, we did not observe hyperfiltration, and there were no differences in the eGFR between them and healthy controls. We found a negative correlation between NGAL and KIM-1, but it was not statistically significant. It was probably because diabetic changes in the kidneys were not advanced.
We are planning a further multicentre study, in which we will analyse markers of tubular damage in babies born with large birth weight because of hyperglycaemia caused by diabetes in pregnancy.
In conclusion, urine NGAL/cr. is statistically significantly higher in newborns exposed to maternal diabetes during pregnancy compared to babies from normal pregnancies. It may suggest subclinical tubular dysfunction in these babies. Urine NGAL is a more sensitive marker than KIM-1 in these newborns. The level of urine NGAL is higher in female babies than in male newborns. In children with normal birth weight born from diabetic pregnancies, eGFR is normal and we do not observe hyperfiltration.