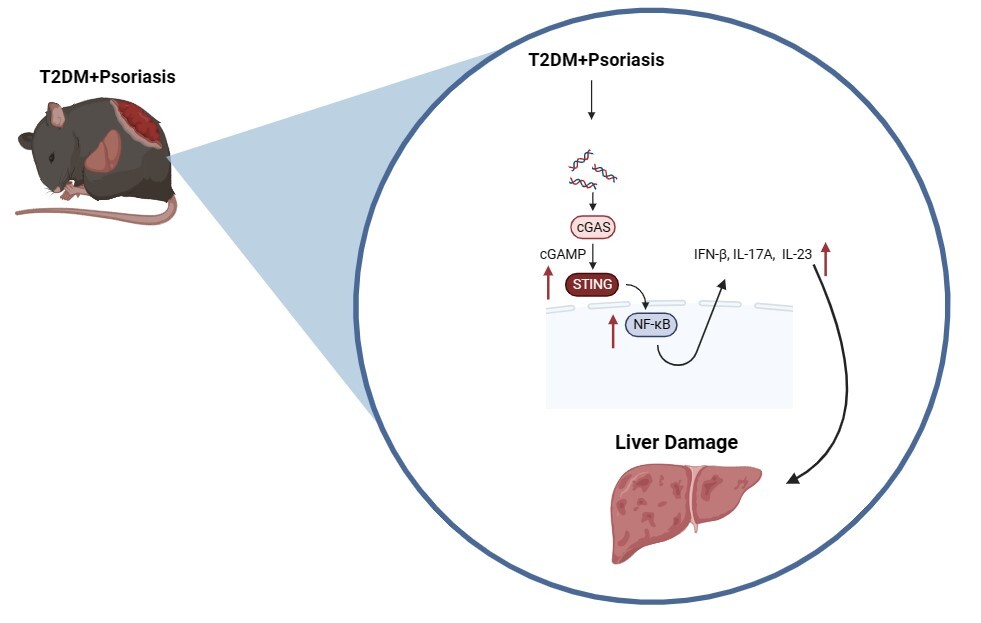
Introduction
Psoriasis is a chronic and common inflammatory skin disease that presents symptoms such as erythema, scaling, and itching on the skin surface [1]. Previous research conducted over 14 years in patients with psoriasis showed a significantly increased risk of developing type 2 diabetes mellitus (T2DM) regardless of factors such as age, body mass index (BMI), smoking, and alcohol intake [2]. It is suggested that psoriasis and T2DM may share inflammatory pathways in their pathogenesis, which could explain the co-occurrence of these two conditions.
Inflammation is a natural bodily response that is essential for fighting infections and promoting tissue repair. However, chronic and uncontrolled inflammation can also contribute to the development and progression of inflammatory disorders. Individuals with psoriasis often exhibit an exaggerated inflammatory response, which may contribute to the onset of T2DM [3]. On the other hand, T2DM is often linked to chronic low-grade inflammation, which can create a favorable environment for the development of psoriasis and may contribute to its progression [4]. Recent research has shown a strong correlation between psoriasis and metabolic disorders such as obesity and liver damage [5, 6]. Additionally, the coexistence of psoriasis and T2DM can result in more severe liver damage [7]. For example, TNF-α is an inflammatory mediator that is secreted by dendritic cells when they are activated in an inflammatory environment. It induces the expression of inflammatory factors and adhesion molecules that are associated with psoriasis and T2DM. Additionally, it contributes to pro-inflammatory factors that trigger inflammatory responses in the liver [8]. Investigating novel treatment strategies for psoriasis that focus on reducing systemic inflammation may help to prevent or reverse the inflammatory damage associated with the coexistence of psoriasis and diabetes, ultimately mitigating liver injury.
Interferon gene-stimulating factor (STING) is a protein that regulates inflammation and is involved in the progression of inflammatory conditions, including liver fibrosis and nonalcoholic steatohepatitis [9]. Studies have found a correlation between the expression level of STING and the stage of liver fibrosis [10]. Activated STING is thought to trigger the classical nuclear factor κB (NF-κB) pathway, thereby regulating the production of interferons and inflammatory factors and modulating hepatic inflammatory responses. Our previous research suggested the potential involvement of the STING pathway in the cutaneous inflammatory response observed in mice with psoriasis and T2DM [11]. However, the function and mechanism of the STING pathway in liver damage associated with psoriasis and type 2 diabetes mellitus are still unclear. Further studies are necessary to explore the specific role and mechanism of the STING pathway in liver damage in the context of psoriasis and T2DM.
In this study, we aimed to expand upon previous research by collecting additional clinical data and using imiquimod cream to induce psoriasis-like skin lesions in T2DM mice. Our objective was to investigate whether the coexistence of psoriasis and T2DM exacerbates liver damage, and to elucidate the potential role of STING and its mechanism in the development of liver damage associated with psoriasis and T2DM.
Material and methods
Collection of clinical data
In this study data were collected from healthy individuals, patients with psoriasis, patients with T2DM, and those with both conditions seeking treatment at the 900th Hospital of the United Nations Peacekeeping Force between December 2017 and December 2022. The collected data included variables such as age, BMI, glycated hemoglobin (HbA1c), alanine transaminase (ALT), aspartate transaminase (AST), Psoriasis Area Severity Index (PASI), and skin immunohistochemistry data. The inclusion and exclusion criteria for participants were based on previous studies conducted by our research team [12]. The study included male and female patients between the ages of 18 and 75 who had been diagnosed with T2DM according to WHO standards (1999) and confirmed psoriasis through clinical and histopathological examination. Patients who had not received treatment or had been treated with the same oral glucose-lowering drugs for at least 3 months prior to inclusion were eligible. Exclusion criteria included patients with severe adverse reactions, type 1 diabetes, pregnancy and lactation, a history of malignant tumors, and serious heart, lung, or kidney conditions, as well as a previous history of pancreatitis. Before participation, all individuals provided written consent, and the Ethics Committee of the 900th Hospital of the United Services Security Force approved the project (Approval No. SC-2017-007). The clinical trial was registered under registration number ChiCTR1800015296.
Construction of the animal model
Construction of the T2DM mouse model
Six-week-old C57BL/6 mice, free of pathogens, were housed individually in cages in a clean-grade environment at a room temperature of 24 ±2°C and a relative humidity of 55 ±10%. Mice were exposed to a daily light cycle from 0700 to 1900 h. They were provided with food and water ad libitum. After 1 week of acclimatization, the mice were randomly divided into normal and high-fat diet groups. The Animal Experimentation Center ensured the welfare of mice. After being fed a high-fat diet for 12 weeks, the high-fat diet group received an intraperitoneal injection of streptozotocin (STZ), whereas the normal diet group received an intraperitoneal injection of the same dose of citrate buffer as the control group. After 7 days, mice with a fasting glucose level ≥ 11.1 mmol/l for three consecutive days were considered to have successfully developed the T2DM model. Two mice from the same normal group and those with T2DM were randomly selected for anatomical observation. Liver histopathological sections were used to determine the success of the liver injury model. An intraperitoneal glucose tolerance test (IPGTT; 2 g/kg) was conducted, and glucose levels in the tail vein blood were continuously monitored at 0, 30, 60, 90, 120, and 150 min after injection. The area under the curve (AUC) was calculated using the trapezoidal rule. This experiment was reviewed and approved by the Animal Ethics Committee of our hospital (Approval No. 2020-059). All experimental feeding and sampling operations strictly adhered to the regulations governing the management and protection of experimental animals.
Construction of a mouse model of T2DM with psoriasis
In the established T2DM mouse model, mice were anesthetized with chloral hydrate. The hair on their backs was shaved and the skin was exposed. Imiquimod cream (62.5 mg) was evenly applied to the exposed skin area on the backs of the mice using a rubber mallet. Petroleum jelly (62 g) was used. In the control group, 5 mg/pc control cream was applied to the exposed skin area on the backs of the mice. The skin tissues in the exposed area were observed with the naked eye in a single-blind manner, ensuring an unbiased evaluation of the study outcomes. Throughout the experimental period, the mice were observed without any obstruction and photographed. The PASI values, which indicate erythema, desquamation, and skin thickening in the exposed skin tissues on their backs, were recorded daily. These indicators were evaluated on a four-point scale, with grades of 0 (absent), 1 (mild), 2 (moderate), 3 (severe), and 4 (very severe). Subsequently, scores for the three indices were calculated individually, resulting in a range of 0 to 12 points. After seven consecutive days of imiquimod cream application, the mice were euthanized. Blood samples were collected from the eye sockets and hearts, and skin and liver tissues were extracted and stored at –80°C. A small amount of tissue was fixed in 4% paraformaldehyde for histopathological examination.
Normal chow diet mice and mice with type 2 diabetes were randomly selected and assigned to five groups. These groups included the following: NC group (normal chow diet + Vaseline), psoriasis group (normal chow diet + imiquimod), T2DM group (type 2 diabetes + Vaseline), psoriasis + T2DM group (type 2 diabetes + imiquimod), and STING inhibitor group (type 2 diabetes + imiquimod + C-176). In the STING inhibitor group, the mice with type 2 diabetes were administered 7.5 µl (750 nmol) of the STING-specific inhibitor C-176 based on the established model. The remaining groups received an equivalent amount of dimethyl sulfoxide (DMSO) mixed with corn oil as a control. This control solution was administered intraperitoneally once daily for 3 weeks (Supplementary Figure S1).
Primary reagents in the experiment
The following products from different suppliers were used in the experiment: imiquimod cream from Mingxinlidi (China), TMEM173/STING antibody from Proteintech (China), TNF-α antibody from Abclonal (China), IL-1β antibody from Abclonal (China), IL-17A antibody from CST (USA), IL-23 antibody from CST Inc. (USA), NF-κB p65 antibody from CST (USA), IFN-β antibody from Abcam (USA), β-actin monoclonal antibody from Sigma (USA), (STZ) from Sigma (USA), C-176 (STING-specific inhibitor) from MCE (USA), and corn oil from MCE (USA).
Detection of serological indicators
After the mice were euthanized, 3–5 ml of blood samples were obtained from the eyes and heart. The samples were then placed in procoagulant tubes and kept at room temperature for 2 h or at 4°C overnight to precipitate serum. The centrifuge was centrifuged at 1000 rpm for 20 min at 4°C, after which the supernatants were collected and transferred to new EP tubes. Finally, the samples were stored at –80°C. Mouse serum was analyzed for ALT, AST, total cholesterol (TC), and triglyceride (TG) using an Olympus AU2700 automatic biochemistry instrument. The IFCC method was used to measure TG, ALT, and AST levels.
Pathological sections and hematoxylin-eosin (HE) staining
Skin and liver tissue sections were prepared by degreasing and washing. This was followed by staining with hematoxylin to visualize cellular components. The tissue was then acid-washed to enhance the contrast and subsequently rewashed. Eosin staining was used to highlight the cytoplasmic structures. After another wash, the tissue sections were dehydrated, cleaned, and covered with slides, allowing observation under a light microscope for further analysis.
Immunohistochemical (IHC) staining
Antigens were retrieved to enhance antigenicity and prepare the skin and liver tissue sections for immunofluorescence microscopy. Non-specific binding was blocked to minimize background noise. The sections were incubated with primary antibodies specific to the target proteins of interest. After removing the excess primary antibodies, the sections were incubated with secondary antibodies conjugated with fluorophores. Another round of washing was conducted to remove unbound secondary antibodies. A reaction substrate was applied to visualize the fluorescence signal, followed by washing to remove the excess substrate. Finally, the sections were sealed to prevent dehydration. Immunofluorescence microscopy was used to visualize protein expression in skin and liver tissues. The stained sections were observed under a fluorescence microscope and representative images were captured. To quantify protein expression, mean optical density values were calculated based on the staining intensity and positive area using ImageJ software. This allowed for measurement and comparison of protein levels between different groups or conditions.
Western blotting
The liver tissue protein samples were subjected to cell lysis and protein extraction. Subsequently, the samples were separated by polyacrylamide gel electrophoresis (SDS-PAGE). An appropriate gel concentration was selected based on the size of the proteins. After separation, the proteins were transferred onto polyvinylidene fluoride membranes (PVDF). The separated proteins were transferred from the gel to the membrane using an electrophoretic transfer device and transfer buffer for electrophoretic transfer. The membrane was then incubated with a primary antibody targeting a specific protein. Subsequently, the membrane was incubated with a secondary antibody that specifically binds to the primary antibody. The ECL developing solution was prepared according to the manufacturer’s instructions, and the automated chemiluminescence image analysis system was utilized to develop the color of each band in the solution for 1–2 min. Subsequently, it was promptly transferred to the exposure instrument to examine and analyze the target protein bands.
Statistical analysis
All statistical analyses were performed using SPSS version 28.0. The results are presented as mean ± standard deviation (SD). The normality of the data was assessed using the Shapiro-Wilk test, while the homogeneity of variance was assessed using the Levene test. For normally distributed data, comparisons between groups were conducted using one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) test for post-hoc analysis. For non-normally distributed data, the Kruskal-Wallis test was used for group comparisons. Statistical significance was determined at a p-value of less than 0.05 (p < 0.05).
Results
Liver function indices and STING expression in patients with psoriasis and T2DM
In Table I, the comparison of demographic and metabolic parameters among the four groups (healthy population, psoriasis, T2DM, and psoriasis in T2DM) showed no significant differences in age, BMI, triglycerides, and cholesterol levels (p > 0.05). However, HbA1c levels were significantly higher in the T2DM group and psoriasis in the T2DM group compared to the healthy population and psoriasis alone (p < 0.01). There were no statistically significant differences between the T2DM groups and the psoriasis with the T2DM group (p > 0.05). When comparing liver function indices among the four groups, the psoriasis combined with T2DM group had higher AST and ALT levels (p < 0.05).
Table I
Demographic and clinical characteristics
| Parameter | Normal (n = 10) | Psoriasis (n = 10) | T2DM (n = 10) | Psoriasis + T2DM (n = 10) |
|---|---|---|---|---|
| Age [years] | 44.8 ±5.4 | 44.4 ±6.2 | 46.2 ±5.4 | 48.2 ±6.7 |
| BMI [kg/m2] | 23.1 ±2.6 | 24.6 ±2.4 | 24.2 ±1.9 | 24.8 ±1.1 |
| HbA1c (%) | 5.9 ±0.5 | 6.0 ±0.5 | 8.0 ±0.7**&& | 8.1 ±0.9**&& |
| Triacylglycerols [mmol/l] | 1.9 ±0.7 | 1.9 ±0.3 | 2.2 ±0.9 | 2.0 ±0.5 |
| Total cholesterol [mmol/l] | 4.5 ±0.7 | 4.7 ±0.4 | 4.8 ±0.7 | 5.0 ±0.8 |
| AST [U/l] | 23.0 ±6.7 | 21.4 ±8.6 | 24.8 ±6.3 | 34.6 ±9.6*&# |
| ALT [U/l] | 22.6 ±5.7 | 24.6 ±4.5 | 26.2 ±6.6 | 35.4 ±8.0**&# |
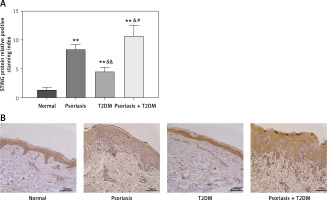
In Figures 1 A and B, the expression of STING protein in the skin tissue was examined in each group. The results showed that patients with psoriasis, T2DM, and psoriasis with T2DM had significantly higher STING protein expression levels than those in the control group (p < 0.01). The study also found statistically significantly higher protein upregulation in patients with psoriasis and T2DM compared with those with psoriasis or T2DM alone (p < 0.05).
Figure 1
Clinical indicators of patients with psoriasis and T2DM. A, B – STING protein levels in the skin tissue of patients were measured by immunohistochemistry (×200). Scale bar = 50 μm. Data are presented as n (%) or mean ± SD. *P < 0.05 vs. NC, **p < 0.01 vs. NC, &p < 0.05 vs. Psoriasis, &&p < 0.01 vs. Psoriasis, #p < 0.05 vs. T2DM, ##p < 0.01 vs. T2DM
Comparison of body weight, blood glucose, blood lipid and liver function indexes in each group
In this study, a T2DM mouse model (DM group) was established by administering a high-fat diet and STZ. After a 12-week intervention, the results showed significant increases in fasting blood glucose (FBG) levels (Figure 2 A) in the DM group compared with the normal control (NC) group. The IPGTT and AUC analyses (Figures 2 B, C) demonstrated impaired glucose tolerance in the DM group compared to the NC group (p < 0.05).
Figure 2
Comparison of the basic indices of mice in each group. A – Comparison of fasting blood glucose (FBG) levels among groups of mice. B – Intraperitoneal glucose tolerance test (IPGTT) (2 g/kg) was performed by measuring blood glucose levels in the tail vein and area under the curve (AUC). C–G – Assessment of serological indices in groups of mice. Data are presented as n (%) or mean ± SD. NC – normal control, PS – psoriasis, DM – T2DM, PS + DM – psoriasis + T2DM. Compared to the NC group, *p < 0.05, **p < 0.01. Compared to the PS group, &p < 0.05
Serological index outcomes showed significantly elevated TG and TC levels in the DM and psoriasis combined with T2DM (PS + DM) groups compared to those in the NC and psoriasis (PS) groups (Figures 2 D, E) (p < 0.05). These findings suggest that the DM group of mice exhibited abnormal lipid metabolism. Compared to the NC group, the levels of ALT and AST gradually increased in the PS, DM, and PS + DM groups, with the most notable elevation observed in the PS + DM group (Figures 2 F, G). This indicates that the combination of psoriasis and diabetes exacerbates impaired hepatic function in mice.
Comparison of back skin lesion tissues of mice in each group
Naked-eye examination of the skin lesions on the backs of mice after hair removal (Figures 3 A, B) showed that the exposed skin tissue of the NC and DM groups appeared healthy without any visible abnormalities, as indicated by a PASI of zero. In contrast, the skin tissues of the PS and psoriasis with T2DM (PS + DM) groups exhibited varying degrees of erythema (redness), scaling, and skin thickening. These groups also had significantly higher PASI values than the NC and DM groups (p < 0.01). Specifically, in the PS + DM group, the skin tissue lesions were more severe on the back and the PASI was significantly higher than that in the psoriasis alone (PS) group (p < 0.01).
Figure 3
Comparison of skin lesions in each group of mice. A – Visual changes in the back skin of each group. B – Comparison of PASI of the back skin of mice in each group. C – Histopathological changes were observed under a light microscope after HE staining (×200 magnification). D – The number of inflammatory cells in the dermis was analyzed. Data are presented as n (%) or mean ± SD. NC – normal control, PS – psoriasis, DM – T2DM, PS + DM – psoriasis + T2DM. Compared to the NC group, *p < 0.05, **p < 0.01. Compared to the PS group, &p < 0.05, &&p < 0.01. Compared to the DM group, #p < 0.05, ##p < 0.01
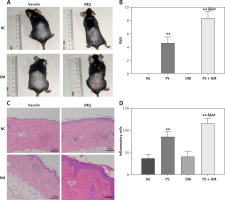
Histological examination of skin tissue at the dorsal lesions in mice using HE staining revealed thickening of the skin tissue in both the PS and PS + DM groups (Figures 3 C, D). This thickening is characterized by an increase in keratinized and epidermal cell layers. Additionally, there was significant infiltration of inflammatory cells in the dermis, indicating inflammation. Compared with the NC and DM groups, the PS and PS + DM groups exhibited significant histopathological changes and increased inflammatory cells in the skin lesions on the back. Notably, the skin lesions in the PS + DM group showed more pronounced histopathological changes and a higher number of inflammatory cells than those in the PS group. These findings confirmed the successful establishment of psoriasis in a T2DM mouse model.
Psoriasis with T2DM aggravates liver pathological damage in mice
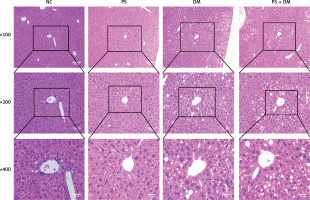
HE staining of liver tissues demonstrated (Figure 4) that mice in the NC and PS groups presented with normal liver tissue architecture, featuring clear visibility of hepatic lobules, radial arrangement of the cords of hepatocytes, and an absence of lipid vacuoles within the hepatocyte cytoplasm. In contrast to the preceding groups, the liver cells of mice in the DM and PS + DM cohorts showed significant enlargement, cytoplasmic edema, and reduced density. Marked lipid droplets were observed within cells, with predominant vesicular lipoatrophy. The nuclei were positioned at the cell periphery, and some hepatocytes displayed nuclear condensation and dissipation. In addition, inflammatory cell infiltration was infrequently observed. Further comparison between the groups with diabetes mellitus (DM) and those with both psoriasis and T2DM (PS + DM) demonstrated that the extent of liver damage was more pronounced in PS + DM mice.
STING and its downstream inflammatory pathways are involved in liver injury in mice with psoriasis and T2DM
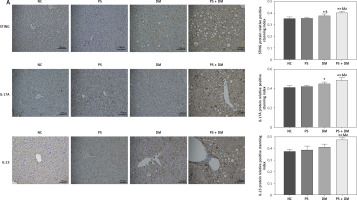
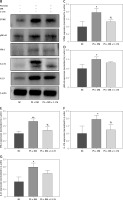
Immunohistochemical analysis of liver tissues from mice indicated that the protein expression levels of STING, IL-17A, and IL-23 were elevated in the PS, DM, and PS + DM groups relative to those in the NC group, with the PS + DM group exhibiting the most significant upregulation (Figure 5 A).
Figure 5
Expression levels of target proteins in the liver tissue of mice in each group. A – Immunohistochemical detection was used to measure the protein expression levels of STING, IL-17A, and IL-23 in the liver tissues of the mice in each group. B–G – WB blot analysis of STING, IL-17A, and IL-23 protein expression levels in the liver tissues of mice in each group. Scale bar = 100 μm. Data are presented as n (%) or mean ± SD. NC – normal control, PS – psoriasis, DM – T2DM, PS + DM – psoriasis + T2DM. Compared to the NC group, *p < 0.05, **p < 0.01. Compared to the PS group, &p < 0.05. Compared to the DM group, #p < 0.05

Western blot analysis demonstrated increased STING protein expression and elevated expression of inflammatory factors such as NF-κB p65, IFN-β, IL-17A, and IL-23 in mice from the PS, DM, and PS + DM groups. Notably, mice in the PS + DM group exhibited the most significant upregulation of these proteins (Figure 5 B–G). These results imply that STING and its associated downstream inflammatory pathway may play a role in the inflammatory response to liver damage in mice with psoriasis and type 2 diabetes.
STING-specific inhibitor C-176 ameliorates skin and liver damage in mice with psoriasis and T2DM
To investigate the impact of inhibiting STING expression on liver damage in mice with psoriasis and type 2 diabetes, we administered the STING-specific inhibitor C-176 to mice with psoriasis and T2DM (PS + DM). Administration of C-176 blocked the expression of STING and determined whether inhibiting STING could reduce inflammatory responses and mitigate skin and liver damage. The back lesions of the mice were observed without magnification. After 3 weeks of C-176 intervention, the back lesion tissues of mice in the PS + DM + C-176 group showed improvement in erythema, scaling, and skin thickening compared to those in the PS + DM group. Additionally, PASI demonstrated a reduction in skin symptoms following the C-176 intervention (p < 0.05) (Figure 6 A, B).
Figure 6
The STING-specific inhibitor C-176 ameliorates skin lesions and liver damage in mice with psoriasis combined with T2DM. A – Visual changes in the skin on the back. B – Comparison of PASI in the back skin of the mice. C – Histopathological changes were observed under a light microscope after HE staining (×200 magnification). D – The number of inflammatory cells in the dermis was analyzed. E – HE staining of mouse liver tissue. PS + DM group – psoriasis + T2DM group. PS + DM + C-176 group – psoriasis + T2DM + C-176 group. Compared to the PS + DM group, %p < 0.05, %%p < 0.01
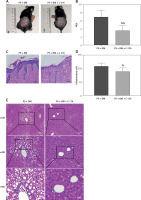
In the PS + DM + C-176 group, HE staining of the liver revealed significantly less hepatocyte swelling (edema), a lower number of lipid vacuoles in the liver cells (cytoplasmic lipid vacuoles), and lower inflammatory cell infiltration in the confluent region of the liver compared to the PS + DM group (Figure 6 E). These findings indicate that administration of the STING-specific inhibitor C-176 effectively mitigated liver damage in mice with psoriasis and type 2 diabetes.
C-176 improves the inflammatory response to liver damage in mice with psoriasis and T2DM by inhibiting the STING inflammatory pathway
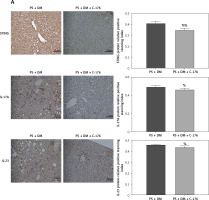
Immunohistochemical analysis of liver tissues showed that in mice treated with C-176, the relative protein expression of STING, IL-17A, and IL-23 was lower than that in the PS + DM group (Figure 7 A). Additionally, the results of western blot assays revealed that protein expression levels of STING and inflammatory factors such as NF-κB p65, IFN-β, IL-17A, and IL-23 were significantly lower in the liver tissues of mice that received PS + DM + C-176 treatment than in the PS + DM group mice (Figures 7 B–G). These findings suggest that the inhibition of STING activation through the STING-specific inhibitor C-176 can reduce the inflammatory response caused by liver damage in mice with psoriasis and type 2 diabetes.
Figure 7
The STING-specific inhibitor C-176 ameliorates the inflammatory response in the liver of mice with psoriasis combined with T2DM. A – Immunohistochemical detection was used to measure the protein expression levels of STING, IL-17A, and IL-23 in the liver tissues of the mice in each group. B–G – WB blot analysis of STING, IL-17A, and IL-23 protein expression levels in the liver tissues of mice in each group. Scale bar = 100 μm. Data are presented as n (%) or mean ± SD. NC group – normal control group. PS + DM group – psoriasis + T2DM group. PS + DM + C-176 group – psoriasis + T2DM +C-176 group. Compared to the NC group, *p < 0.05, **p < 0.01. Compared to the PS + DM group, %p < 0.05
Discussion
Psoriasis is an autoimmune disease that primarily affects the skin [13]. However, it is also closely associated with metabolic disorders such as obesity, dyslipidemia, cardiovascular disorders [14], and nonalcoholic fatty liver disease [15]. The coexistence of psoriasis and metabolic liver disease is common in clinical practice [16]. Not only is metabolic-associated fatty liver disease diagnosed more often in patients with psoriasis, but its clinical course is also more aggressive [17]. The underlying mechanisms and treatment options for this combination are not yet fully understood. Psoriasis and T2DM share common pathophysiological characteristics including inflammation [18], obesity, and insulin resistance [19]. Both diseases create a complex pathological environment that affects multiple organs in the body. The liver is particularly susceptible to damage owing to a combination of various pathological mechanisms, which poses a significant challenge for clinical treatment. Therefore, it is essential to investigate the mechanisms underlying liver damage in individuals with psoriasis and T2DM to identify potential therapeutic targets. In this study, we aimed to explore the role and potential mechanisms of liver damage in individuals with psoriasis and T2DM, using clinical data and animal experiments.
This study revealed higher liver function indices in individuals with psoriasis and T2DM than in those with psoriasis or diabetes alone. This finding suggests that the coexistence of these two diseases may increase the risk of liver damage. To further investigate liver damage associated with the combination of psoriasis and diabetes, a mouse model of psoriasis and T2DM was developed. Mice with both psoriasis and diabetes exhibit a significant increase in skin and liver damage. Both clinical and animal studies have indicated that the STING protein, located on the endoplasmic reticulum membrane, and its downstream inflammatory pathway may play a role in the development of skin and liver damage in individuals with psoriasis and type 2 diabetes. The STING pathway can be activated by endoplasmic reticulum stress and mitochondrial injury [20–22]. By connecting upstream DNA sensors to downstream IRF3 and NF-κB pathways, STING plays a critical role in the innate immune response and defense against viral damage by inducing the expression of IFN-β and inflammatory factors [23]. In recent years, STING inhibitors have shown potential in preventing and treating inflammatory and autoimmune diseases, including infectious diseases, psoriasis, systemic lupus erythematosus, and non-alcoholic fatty liver disease [24, 25].
Why is STING pathway activation observed in mice with psoriasis and diabetes? In psoriasis mouse models, imiquimod has been found to produce large amounts of ROS via the inhibition of mitochondrial complex I [26]. Oxidative stress due to abnormal glycolipid metabolism in keratinocytes can also cause ROS overload, leading to mitochondrial dysfunction and mtDNA fragmentation [21, 27]. The mitochondrially derived DNA (mtDNA), packaged in an anomalous manner, is released into the cytoplasm. The cytoplasm detects it via a cGAS DNA sensor, which triggers the STING pathway through inflammatory vesicles and sets off cascading inflammatory responses [20, 28]. In contrast, diabetes involves abnormal glucose-lipid metabolism, which can lead to necrosis or apoptosis of hepatocytes in the liver due to lipid oxidative stress. This can result in nuclear DNA or mtDNA release, potentially activating STING signaling and the apoptotic pathway in hepatocytes through DNA-mediated production of IFN-I, which ultimately leads to aseptic inflammation [29]. The activation of STING in macrophages of monocyte origin and Kupffer cells in the liver, where activation contributes to the phosphorylation of NF-κB, produces TNF-α and IL-1β to initiate inflammatory pathways in hepatocytes [10]. It also triggers the activation of TGF-β1 in stellate cells, triggering stem cells, leading to fat deposition and liver fibrosis [30].
Inflammation has been suggested as an intrinsic mechanism in the development of diseases such as metabolic syndrome, insulin resistance, and diabetes, and it is also strongly associated with psoriasis, depression, cancer, and kidney disease, among others [31]. The role of IL-17 as a proinflammatory cytokine of the adaptive immune system has received considerable attention. It is produced by a new T helper cell subset known as “Th17” [32]. Our study showed a significant increase in the expression of STING and inflammatory factors, including NF-κB p65, IFN-β, IL-17A, and IL-23, in the liver tissues of mice with induced psoriasis and T2DM. This suggests that the STING pathway is involved in the inflammatory response to the liver damage caused by the combination of psoriasis and T2DM. Additionally, we examined the effects of C-176, a specific inhibitor of the STING pathway, on the livers of mice with psoriasis and T2DM. C-176 treatment decreased inflammatory cell infiltration and significantly reduced the expression of downstream proteins and inflammatory factors. These findings suggest that inhibition of the STING pathway may alleviate the inflammatory response and liver damage in individuals with psoriasis and T2DM. Moreover, the antiviral medication remdesivir has been found to suppress STING signaling and reduce inflammation and lipid dysfunction in metabolic liver disease, making it a potential therapeutic option [33]. This further highlights the potential of targeting the STING pathway to treat liver damage in patients with psoriasis and T2DM.
Despite the valuable insights gained from this study, certain limitations should be acknowledged. First, the use of imiquimod-induced psoriasis lesions in animal models was limited to approximately 1 week, which may restrict the understanding of the pathogenesis of psoriasis in target organs in animal studies. Additionally, no prior reports have described the construction of animal models of psoriasis combined with T2DM through single- or double-gene knockdown. Furthermore, the expression of STING in the liver is controversial. Some studies have suggested that STING can be expressed and activated in hepatocytes [29, 34], while others have proposed that STING expression and activation occur solely in hepatic immune cells [35]. Further research is needed to determine whether it primarily affects intrinsic liver immune cells or is mediated through blood-derived immune cells at the single-cell level to elucidate the impact of STING signaling on the innate immune response in the liver. Addressing these limitations through future studies will provide a more comprehensive understanding of the role of STING in the liver damage associated with psoriasis and T2DM. In the section on clinical studies, the sample size was too small. Currently, we did not use STING inhibitors available for the clinical treatment of patients with diabetes and psoriasis-associated liver injury, and there are no STING inhibitors available for clinical treatment of patients with diabetes and psoriasis-associated liver injury. Future research could explore the potential of existing glucose-lowering drugs or psoriasis medications that can target the inhibition of the STING inflammatory pathway. This could delay and treat liver injury while improving psoriasis symptoms.
In conclusion, the findings of our clinical trials and animal studies provide evidence that the STING pathway is involved in the inflammatory response to liver damage in individuals with psoriasis and T2DM. Additionally, inhibition of STING activation has shown potential in reducing the inflammatory response in the liver of individuals with psoriasis and T2DM, suggesting that it could be a therapeutic target. It is important to note that further research is needed to address certain limitations and to fully understand the role of STING in liver damage associated with psoriasis and T2DM. Nonetheless, these findings provide valuable insights into the potential mechanisms and treatment options for liver damage in patients with psoriasis and T2DM.