Introduction
Gigantomastia is a rare condition characterised by excessive breast growth. It can be physically disabling for a patient due to mastalgia, neck and back pain, headaches, trophic lesions of the breast skin with ulceration and infection, difficulty finding well-fitting clothes, and limited ability to exercise. It can also cause psychosocial problems, such as depression and sociophobia, related to the cosmetic defect. To date, there has not been a universal classification or definition for gigantomastia. It used to be defined as breast enlargement that requires reduction of over 1500 g per breast; however, currently physical and psychological symptoms seem to be the major criteria for the diagnosis rather than the volume of excess breast tissue that needs to be removed [1–5].
The pathophysiology of mammary enlargement varies depending on the type of gigantomastia. It can be the result of hormonal disturbances and changes due to some disorders or physiological conditions like pregnancy – gestational gigantomastia (GG) [6–8]. The aetiology of GG remains elusive. Although some theories have been proposed, including excessive production of oestrogen or prolactin, hormone receptor sensitivity, or underlying autoimmune disease triggered by pregnancy, none was scientifically verified [9, 10]. It was also hypothesised, that GG in women with normal hormone levels (the most common type) may be explained by increased hormonal sensitivity in the target organ, but this theory was not verified [11–13].
The second most common type of gigantomastia is juvenile virginal enlargement of the breast, which can be seen as early as in late childhood and is often present between the ages of 11 and 14 years [1, 14]. Some authors also identify spontaneous idiopathic gigantomastia, defined as breast enlargement not related to puberty or pregnancy [15, 16]. These are, however, case-reports, which makes it difficult to recognise this type of gigantomastia. Moreover, recent case-studies show that the reason for such a presentation may be pseudoangiomatous stromal hyperplasia (PASH) [17, 18].
The aim of this research was to examine the receptor status (oestrogen receptor α (ERα) and progesterone receptor (PR)) of breast tissue in adult women with juvenile or idiopathic gigantomastia.
Material and methods
Participants
The study involved 70 patients who underwent breast reduction due to juvenile or idiopathic gigantomastia in two plastic surgery centres located in different regions of the country (27 women from one centre and 43 from the other). The average age of the studied individuals was 39.9 ±10.35 years (range: 19–59). All patients qualified for surgical treatment had undergone endocrine examinations, and a detailed medical interview had also been conducted to exclude other possible reasons for breast enlargement. Breast ultrasonography or mammography examinations were performed. All procedures were carried out because of therapeutic indications and were financed by the National Health Fund. To meet these criteria patients provided referrals from a neurosurgeon, neurologist, or orthopaedist confirming a cervical or spine disorder due to heavy breasts. Exclusion criteria included: any hormonal disturbances or treatment (current or past, excluding contraceptives), obesity (BMI > 30 kg/m2), pregnancy-related gigantomastia, operation disabling general state of health, any abnormalities in breast imaging, history of breast malignancy, no clinical physical symptoms of gigantomastia, and aesthetic reasons. Surgical techniques involved breast reduction with nipple-areola complex transposition with upper, lower, or upper-medial pedicles or as a free graft. Histopathological examinations of removed tissues were done routinely. Tissue samples for the examination of receptors were obtained after a histopathological analysis. ERα and PR expressions were detected immunohistochemically. This examination was done in the case of all samples from both clinics, in one centre, by one histopathology specialist.
The control breast samples were obtained from 18 female cadavers (mean age ± SD: 66.3 ±10.52 years) on whom obligatory post-mortem examinations were performed due to national law. A sample of breast gland was harvested by a histopathology specialist, in addition to the routine procedure, for the purpose of the study. The specialist excluded women who had a history of breast cancer or had macromastia.
The protocol for the study was approved by the Local Ethics Committee (of the Medical University of Lodz, RNN/191/17/KE).
Immunohistochemistry
Immunohistochemical staining for ERα and PR was performed on 3 μm sections from archive paraffin blocks. The sections were deparaffinised and antigen retrieval was performed using DakoTPlink. The slides were then loaded on a Dako Autostainer Plus (the elements of the automatic Dako line for standardised immunohistochemistry) and incubated with primary “ready to use” anti-ERα and anti-PR (PR clone PgR636 binding with PR-A and PR-B) antibodies according to the manufacturer’s instructions. As a positive control, the sections from ER-α and PR-positive breast carcinoma were used. In each case two slides were evaluated and 200 nuclei were counted. The nuclear immunoexpression of ERα and PR was observed in glandular epithelium of breast tissue.
Oestrogen and progesterone receptor concentration measurements
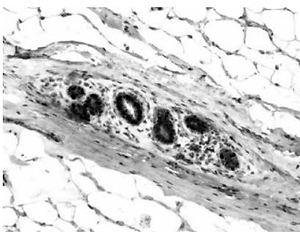
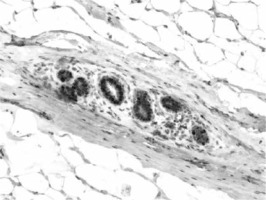
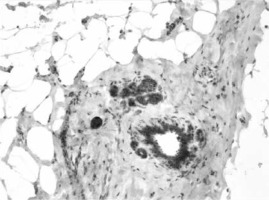
ERα- and PR-positive nuclei were evaluated using a computer image analysis system consisting of a PC computer equipped with a Pentagram graphic tablet, Indeo Fast card (frame grabber, true-colour, real-time), produced by Indeo (Taiwan), and a Panasonic colour TV camera (Japan) coupled with Carl Zeiss microscope (Germany). This system was programmed (MultiScan 18.03 software, produced by Computer Scanning Systems, Poland) to calculate the number of objects (semiautomatic function) (Figures 1 and 2). The results were presented as the percentage of positive nuclei from all nuclei counted in the glandular epithelium.
Statistical analysis
An analysis was conducted in the aspect of the differences in the ERα and PR expression in breast glands in women with gigantomastia and in the control glands. The normality of distribution of the tested variables was examined using the Kolmogorov-Smirnov test. Due to the lack of normality of distribution of data concerning ERα and PR expression, the non-parametric Mann-Whitney test was used. To examine correlations between ERα and PR expression and patients’ age, Spearman’s correlation coefficients were calculated.
For statistical purposes we categorised ERα and PR expression as weak (0–30%), moderate (31–60%), high (61–90%), or very high (91–100%) and compared the categorised data.
Results
All glands were subjected to a routine histopathological examination, which did not reveal any cases of tumours. Categorised and uncategorised ERα and PR expression did not differ between women with gigantomastia and control women. It was found that in both groups weak (0–30%) ERα and PR expression was the most common (Table I). Statistical analysis did not reveal any correlations between ERα and PR expression and the age of the examined women (Table II). Analysis of categorised data also did not reveal any significant correlations between ERα or PR and the women’s age – for the whole group: p = 0.795 (ERα), p = 0.207 (PR), for women with gigantomastia: p = 0.934 (ERα), p = 0.43 (PR), and for control women: p = 0.638 (ERα), p = 0.805 (PR).
Table I
Categorised and uncategorised oestrogen receptor α (ERα) and PR expression in women with gigantomastia and control women
Discussion
The aetiology of gigantomastia is unknown, and exploration of this issue seems to be important because it could help to answer the question about the possible risk of breast cancer in these women. However, it has not been established if gigantomastia increases this risk and if women with this condition should be subjected to any additional or more frequent screenings for breast cancer. Kusano et al. found that for lean women, larger breast size was associated with a higher risk of breast cancer, and Eriksson et al. showed that two single nucleotide polymorphisms, which have previously been associated with breast cancer risk, are also associated with breast size [19, 20]. Other studies have suggested that expression levels of ERα, PR, insulin-like growth factor 1 receptor (IGF-1R), and Ki67 in cancer-free breast tissue may be associated with subsequent breast cancer risk [21–25].
Only a few studies concerning the possible aetiology of gigantomastia can be found in the literature, and their results are inconsistent. Jabs et al. reported negative expression of oestrogen receptors in the breast tissue of all examined women (n = 25) with breast hypertrophy [26], while Sun et al. reported that oestrogen receptor status was different in patients with breast hypertrophy and micromastia patients [27]. The aim of this study was to determine the expression of ERα and PR in breast tissue obtained from women with pubertal and idiopathic gigantomastia. The results revealed that ERα and PR expressions in the glands of these women did not differ from the receptor expressions in control women. Although the mean age of the control group was higher than the age of the examined women, statistical analysis did not reveal a correlation between age and receptor expression.
The presented study has some limitations. Although the number of cases may seem to be small, juvenile and/or idiopathic gigantomastia is a rare condition, and the inclusion criteria were rigorous because we excluded obese women and those with any possible causative factors of breast enlargement. Another important limitation is the fact that the analysed groups differed in age (pre- and post-menopause women), which might have affected the results. However, we did not find correlation between ERα and PR and the age, which provided a rationale for such analysis.
The results of this study appeared to be different from our findings of idiopathic gynaecomastia in men, because we found out that men with idiopathic gynaecomastia present primary “overexpression” of ER and PR, which may cause idiopathic breast enlargement in this group [28]. In women with gigantomastia the cause of breast enlargement seems to be different and not associated with ERα and PR overexpression in breast glands. Also, this condition was not related to hormonal abnormalities or hormonal drug intake because such women were excluded from the studied group. Moreover, all participants had routine histopathological examination of the resected breast tissue performed, and no pathological findings were detected. Other condition that may present as isolated macromastia – excess aromatase syndrome – is a very rare genetic syndrome, and usually some other clinical manifestations are present. On the basis of the conducted studies, it can be presumed that ERα and/or PR in women with gigantomastia may be “oversensitive” to hormones, or there are other receptor and hormone anomalies responsible for breast enlargement (i.e. growth hormone, cytokines). Additionally, apart from the mammary gland, the major component of the enlarged breast is fatty tissue, which seems to be hypertrophic or hyperplastic, so further studies concerning the aetiology of gigantomastia could focus on the cause and type of fat accumulation in breasts.
In conclusion, hypothesis concerning ERα and PR oversensitivity in women with gigantomastia should be verified by looking for specific gene polymorphisms that could cause this condition. Moreover, analysing abnormal sensitivity of receptors to hormones may also be crucial in establishing the increased risk of breast cancer in women with gigantomastia, because receptor polymorphisms causing receptor hypersensitivity related to increased breast cancer risk were detected [29–31].