Liver diseases are among the most important health problems worldwide. In developed countries, overnutrition and alcohol abuse represent the major causes of liver disease, whereas infectious diseases – especially from viruses and parasites – are the main cause in Africa and Asia. Moreover, mortality rates from liver diseases are increasing rather than declining worldwide [1]. About 2% of the world’s population is chronically infected with hepatitis C virus (HCV) [2], while the prevalence of hepatitis B virus (HBV) in the global population is estimated to be 3.7% [3]: these viruses are the most common underlying agents in infection-related cirrhosis.
The availability of a new class of direct antivirals agents (DAA) against HCV is probably going to change the epidemiology of HCV infection. Results from randomized clinical trials (RCT) testing DAA showed a sustained viral response (SVR) rate up to 100% [2], thus making realizable the prospective of HCV global eradication [4]. However, little is known about the evolution of liver disease after viral eradication has been achieved; the complete regression of hepatic fibrosis is probably unachievable [5].
Liver fibrosis, and the subsequent cirrhosis, are the consequences of chronic liver injury due to several causes: viral cytotoxicity, oxidative stress, toxic stress (e.g. drugs, alcohol) and metabolic damage [6, 7].
Curcumin is the most important curcuminoid of turmeric, and it has been identified as the main factor responsible for its biological activities [8], including potent anti-oxidant and anti-inflammatory effects as well as the ability to modulate several signaling mechanisms [9–12]. Oxidative stress plays an important role in many chronic diseases and in carcinogenesis [13–15]. Several studies have shown that curcuminoid compounds can act as free-radical scavengers by reducing lipid peroxidation mediated by free radicals [16–18]. One of the most important discoveries in this field has been the curcumin-mediated suppression of nuclear factor κB (NF-κB), which has a key role in triggering the inflammatory cascade in most chronic illnesses [19–21]. Importantly, NF-κB-dependent gene products also prevent apoptosis and promote cell proliferation, invasion and angiogenesis [22–26].
This review will focus on curcumin’s role in preventing liver damage progression in different liver diseases, by interfering not only with inflammation and fibrosis, i.e. in non-alcoholic fatty liver disease (NAFLD), but also with HCV replication and other mechanisms involved in hepatocarcinoma (HCC) development in HCV-related chronic liver disease.
Curcumin bioavailability
The interest in curcumin for chronic diseases has recently increased due to its anti-oxidant, anti-inflammatory and anti-tumoral properties [27]. However, the pharmacodynamic actions of curcumin described in pre-clinical trials are limited by its unfavorable pharmacokinetic profile. In fact, curcumin showed low absorption due to its poor solubility in water and fast liver and intestinal metabolism, which contribute to its rapid excretion [28]. Moreover, it is highly unstable and rapidly hydrolyzed at physiological pH [29], and undergoes intense hepatic biotransformation (by conjugation and CYP reduction enzymes with prevalent excretion through the bile) [30].
In order to overcome curcumin’s unfavorable pharmacokinetic profile, several formulations have been proposed, including curcumin-loaded nanoparticles [31], complexes with phospholipids [32], microemulsifying [33] and association with drug bioenhancers [34, 35]. Of particular interest is the delivery system of curcumin developed by Sasaki et al. [36] named Theracurmin, which consists of a highly absorptive formulation dispersed with colloidal nanoparticles. Oral administration of Theracurmin guaranteed a value of area under the curve (AUC) 40-fold higher compared with curcumin powder.
Curcumin and liver damage
Inflammation and fibrosis
Activation of hepatic stellate cells (HSCs) promotes liver fibrosis regardless of etiology. The mechanisms of HSC activation in vivo are often investigated with the fibrotic carbon tetrachloride (CCl4) in animal models. In these models, curcumin showed a major role as an inhibitor of HSC activation; it also seems to be able to reduce liver damage, as well as the a-SMA and procollagen expression in the liver, when administered in CCl4-induced liver fibrosis models for 4–8 weeks. The recognized mechanism of action in those models included curcumin’s ability to target multiple sites, such as platelet-derived growth factor-b receptor (PDGF-bR) [37], matrix metalloproteinases (MMPs) [37, 38], tissue growth factor b (TGFb) [39, 40], peroxisome proliferator-activated receptors (PPARc) [41], toll-like receptors (TLRs) [42], apoptotic pathway [43, 44], inflammatory cytokines [41, 42, 45, 46] and microRNAs [47, 48]. Recently, curcumin’s capacity to increase cyclic adenosine monophosphate levels, which leads to an increase in the number of mitochondrial DNA duplicates, has been demonstrated [49].
A growing body of evidence has shown that leptin and its receptor (Ob-R) play an important role in activating HSCs and in the subsequent development of fibrosis. An in vitro study showed that curcumin is able to interrupt leptin signaling that would lead to HSC activation. It exerts this effect by inhibition of phosphorylation of Ob-R and suppression of Ob-R gene expression by stimulating PPARc activity and reducing oxidative stress [50].
Moreover, curcumin appears to be effective in vitro in inhibiting hyperglycemia-induced HSC activation [51]. It has been shown that curcumin suppression of membrane translocation and gene expression of glucose transporter-2 leads to a reduction of intracellular glucose level of HSCs [51]. The same authors reported that curcumin inhibits the insulin receptor substrates (IRS)/PI3K/AKT signaling pathway, which links leptin with the insulin pathway, preventing the translocation of glucose transporter-4. It suppresses the PKA activity while increasing the activity of AMP-activated protein kinase (AMPK), thus increasing the glucokinase activity and subsequently the conversion of glucose to glucose-6-phosphate; the final effect is a reduction of glucose levels in HSCs [52].
Khan et al. [53] reported that curcumin also has anti-glycation properties: advanced glycation end-products (AGEs) are compounds formed by oxidative and non-oxidative reactions between proteins and/or lipids and carbohydrates [54] and their production is increased by hyperglycemia. Aging, chronic renal failure and diabetes facilitate the accumulation of AGEs in tissues and blood and lead to inflammation and liver fibrogenesis [55]. Amadori products are intermediates in the formation of AGEs; their conversion to AGEs is partially caused by oxidative reactions [56]; anti-oxidant agents may thus prevent the deposition of AGEs in tissues [57] which are involved in HSC activation. Several studies have shown the ability of curcumin to reduce HSC activation thanks to its anti-glycation properties [50, 58, 59]. However, there is no evidence for the role of curcumin in regulating gene expression of RAGE and AGE-R1 (which are the two categories of AGE receptors) in vivo. Lipid metabolism is implicated elsewhere in HSC activation, and curcumin appears to be also effective in inhibiting this pathway. In fact, in vitro studies have highlighted the effect of curcumin in increasing intracellular lipid accumulation in HSCs through the induction of lipogenesis-related genes, such as SREBP-1c, PPARc, and C/EBP a, leading to inhibition of HSC activation [60]. In addition, different studies have also shown significant improvement in the lipid metabolism and delay in hepatic fibrosis progression in rats and mice with steatohepatitis treated with curcumin [61–63].
As shown in in vitro models, another curcumin-mediated mechanism of inhibition of HSC activation is the elicitation of AMPK activity, which induces the expression of genes involved in lipid accumulation as well as in the increase of intracellular fatty acids and triglycerides levels [60]. In fact, the HSC activation is facilitated by reduction of lipid storage capacity and intracellular lipid droplets, as well as by suppression of PPARc, C/EBPa, SREBP 1 and other transcription factors [64, 65]. In detail, hepatic AMPK leads to a reduction in glucose output with a subsequent lowering of blood glucose; this effect is exerted by high AMP and low ATP concentrations [66]. AMPK is also believed to regulate lipogenesis and SREBP 1 activation [67]. Moreover, as reported in studies conducted by Kang et al. [68–70], curcumin exerts several effects that also reduce HSC activation: it suppresses the effects of specific protein-1, which is involved in attenuating the expression of SREBP 2, it reduces the gene expression of lectin-like oxidized LDL receptor-1 (LOX-1) and it suppresses the expression of LDL receptor.
Curcumin also has a role in other steps of fibrogenesis when increased extracellular proteins are deposited in the extracellular space of the liver. This deposition is regulated by the action of a group of enzymes called MMPs and their specific inhibitors, tissue inhibitors of metalloproteinases (TIMPs). When this balance is broken by hyperactivation of HSCs, fibrosis occurs. Available evidence showed that curcumin plays a role in downregulation of TIMP-1 and TIMP-2 in vivo [38, 71–73] and in vitro [74], while it upregulates MMP-2 [73], MMP-7 [72], MMP-9 [73] and MMP-13 [38, 72]. This leads to inhibition of HSC activation due to the degradation of one of the main components of the ECM, the fibrillar collagens. Furthermore, another crucial mechanism involved in liver fibrosis is the reduction of PPARc expression in HSCs: in fact, PPARc is highly expressed in inactive HSCs, while its activity is reduced when HSCs are activated both in vivo and in vitro [65, 75, 76]. Curcumin may thus interfere with liver fibrosis due to its role in induction of the expression of PPAR-c, which results in a reduction of vitamin A and lipid-droplet storing capability, deposition of ECM in the extracellular space and expression of a-SMA and type I collagen-α1, as well as in elicitation of cell proliferation and growth. Finally, the curcumin-mediated PPARc activation results in inhibition of trans-activating activity of NF-κB, while – when a specific PPARc antagonist is used – higher HSC proliferation is observed.
Considering that adipokines, such as leptin and adiponectin, have an important role in regulation adipocyte energy metabolism [77, 78], curcumin has anti-diabetic effects through the suppression of NF-κB in adipocytes and the reduction of NF-κB-regulated adipokines. So, curcumin has anti-diabetic effects that could be considered as valuable targets for diabetes mellitus [79].
With regard to clinical trials in humans, a randomized, double-blind, placebo-controlled trial [80] showed that a 12-week fermented turmeric powder (FTP) treatment was associated with a statistically significant decline in both ALT and AST levels in 30 subjects with mild elevated transaminases concentrations at baseline (with the possible speculation of a reduction of the hepatic cell damage by FTP therapy). One limitation of this study was the unknown etiology of the transaminase elevation in the enrolled patients. However, patients with chronic HCV and HBV infections, as well as those with abnormal transferrin saturation, were excluded before randomization (Table I).
Table I
Randomized clinical trials of curcumin treatment in subjects with different liver diseases
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease, and it is associated with the classic clinical components of the metabolic syndrome: obesity, diabetes, dyslipidemia, insulin resistance [81]. These predisposing conditions are highly prevalent in the western countries; hence NAFLD has become a public health concern with an estimated prevalence of 6–35% among adults worldwide [82]. Liver steatosis is recognized as a decisive “first hit” in the pathogenesis of the disease and it is defined by the presence of cytoplasmic triglyceride (TG) droplets in > 5% of hepatocytes in the absence of significant alcohol consumption [83]. Therefore, modifications in dietary and lifestyle habits represent the first therapeutic target to prevent NAFLD or its progression to non-alcoholic steatohepatitis (NASH)/cirrhosis [84]. However, as in other conditions requiring considerable changes in dietary habits, most patients fail to comply with effective lifestyle modifications. The NAFLD is demonstrated to evolve in severe liver fibrosis and cirrhosis. To date, there is no specific drug available on the market able to effectively reverse liver steatosis or slow NAFLD progression. Therefore, there is a growing interest to find effective and safe treatments for this important clinical problem. Natural products have been the subject of increasing attention owing to their multi-target action and overall safety in several conditions [85–88]. Due to the possible protective action that curcumin exerts on the liver, and the beneficial effect shown on the associated conditions of NAFLD, including obesity [89], dyslipidemia [90], and impaired fasting glucose [91], this compound has been considered as a potential therapeutic agent in NAFLD. In recent years, curcumin (and its derivates) has been tested in animal models of NAFLD with results elucidating possible mechanisms of action and paving the way to possible clinical use. In a methionine choline deficient (MCD)-diet-induced NAFLD mouse model, bisdemethoxycurcumin (BDMC), a close curcumin analogue, was investigated for its potential hepatoprotective effect. To this aim, a control group (normal diet), a NAFLD group (MCD diet), and a MCD + BDMC group were studied after a 4-week treatment. Convincing results showed that BDMC was associated with significantly lower circulating aspartate aminotransferase (AST), alanine aminotransferase (ALT) and inflammatory markers (IL-6 and TNF-α) compared to MCD mice. Moreover, TG levels, expressed as mg/total liver tissue, were very similar to the ones measured in the control group. In this study, the BDMC also showed an inhibitory effect on liver lipogenesis by reducing the expression of genes related to fatty acid synthesis, which are generally found upregulated in NAFLD. In a subsequent study, male Sprague–Dawley rats were fed with a high-fat and high-sugar diet for 8 weeks (NAFLD group), then randomly assigned to curcumin by oral gavage (80 mg/kg/day) or an equal volume of cellulose and compared with a control group (normal diet). Biohumoral markers, including ALT, TG, total cholesterol (TC) and HOMA index, as well as liver histology parameters, were consistent with a significant recovery of the clinical features of the NAFLD in curcumin-fed rats. In this study, a specific pathophysiologic effect has been proposed: by measuring adiponectin precursor (ADIPOQ) mRNA, protein expressions and DNA methylation status and by analyzing the correlations between these and changes in TC, TG, ALT and HOMA-I, DNA methylation of ADIPOQ was found to be one of the mechanisms by which curcumin executes its hepatoprotective function in NAFLD [92]. This first study testing curcumin in humans with NAFLD (grades 1–3 in ascending severity, according to liver ultrasonography definition) evaluated the metabolic effects in subjects randomly assigned to a curcumin (1000 mg/day in 2 divided doses) (n = 44) or control (n = 43) group for a period of 8 weeks. Serum levels of total cholesterol, low-density lipoprotein cholesterol, triglycerides, non-high-density lipoprotein cholesterol and also uric acid significantly decreased in the curcumin group [93]. The highest quality trial testing curcumin compounds in subjects with NAFLD was published in 2016 by Rahmani et al. [94] and added a placebo-controlled design and an ultrasound end-point. In this study, 80 subjects of both sexes, selected as in the study presented above, were 1 : 1 randomly assigned to curcumin (500 mg/day of an amorphous dispersion preparation comprising 70-mg curcuminoids) or placebo supplementation for 8 weeks. Most anthropometric and biohumoral parameters (total cholesterol, LDL-C, HDL-C, triglycerides, BMI, body weight, ALT, AST, HbA1c) were found significantly improved after the supplementation in the curcumin group compared with placebo. Similarly, a comparison of liver ultrasonographic findings (severity of liver steatosis on a scale from 1 to 3) at baseline and after 8 weeks revealed a significant improvement in almost 80% of subjects in the curcumin versus 27% in the placebo group. Interestingly, the effect of curcumin in reducing US evidence of liver fat remained significant after adjustment for possible confounding factors, including baseline levels of TG, HbA1c, ALT and changes in body weight.
Although direct comparison between the two human studies available in the literature are limited by the different curcumin posology employed, there are striking similarities in the overall beneficial effect on the metabolic profile in treated subjects. Findings of this randomized controlled trial are also summarized in Table I.
Moreover, a more recent randomized placebo-control trial of 87 subjects confirmed these data [95]. After 8 weeks of curcumin (phytosomal form, at daily dose of 1 g), a reduction in body mass index and waist circumference and an improvement in ultrasonographic liver findings were observed, with a significant reduction of ALT and AST.
In a previous randomized double-blind placebo-control trial of the same group, 117 subjects with metabolic syndrome were randomly assigned to curcuminoids or placebo [96]. After 8 weeks of follow up, supplementation with curcuminoid (at a daily dose of 1 g) and piperine (at a daily dose of 10 mg) combination significantly improved the oxidative and inflammatory status of patients. In particular, there was observed an improvement in serum superoxide dismutase activities and a reduction in concentration malondialdehyde and C-reactive protein. These results are in line with previous studies [97].
Of note, the important role of curcumin in a clinical setting was recently reviewed by an International Lipid Panel expert [98]. In this work, the authors concluded that an evidence-based approach to the use of lipid-lowering nutraceuticals could improve the quality of the treatment, including therapy adherence, and achievement of the LDL-C goal in clinical practice, an important end-point in cardiovascular disease [99–102]. Moreover, in Table II, ongoing or future trials registered at clinicaltrials.gov to be performed with curcumin in metabolic and hepatic diseases are listed.
Table II
Ongoing or future randomized clinical trials of curcumin in metabolic syndrome, non-alcoholic fatty liver disease and other liver diseases (from clinicaltrials.gov)
Curcumin and HCV replication
Kim et al. demonstrated that the induction of heme-oxygenase 1 (HO-1) and the inhibition of PI3K-AKT partially promote HCV replication. HO-1 is a curcumin-induced gene and it is a potential therapeutic protein for the regulation of homeostasis in several diseases [103]. Furthermore, HO-1 inhibits the replication of HCV [104]. In fact, its knock-down partially reverses the curcumin-mediated inhibition of HCV replication and since HO-1 is induced by ROS, HO-1 and ROS may have a role in inhibiting HCV replication [105, 106]. Kim et al. also showed that curcumin may have a role in the suppression of HCV replication by blocking the AKT-SREBP-1 pathway [107]. A recent study showed different mechanisms in curcumin-mediated HCV replication inhibition. First of all, they demonstrated that the main metabolized form of curcumin (THC), derived from the lack of α1β unsaturated ketone groups, is not active in inhibiting HCV replication. Therefore, they postulated that the key structures involved in this HCV inhibition process were the ketone groups; moreover, they discovered that curcumin was able to affect the entry pathway and had no activity against replication or assembly/release of infectious particles. Curcumin seemed to exert its activity directly impairing the ability of the virus to enter the target cells, and the proposed mechanism of action was a modulation of the fluidity of the viral membrane with no effect on the integrity of viral particles. Moreover, curcumin seems to be able to interfere with the cell-to-cell HCV spreading mechanism, and this capability may be correlated with its hydrophobicity by which curcumin could insert among lipids of the viral envelope, thereby increasing its rigidity and thus inhibiting its fusion capabilities.
Curcumin and hepatocellular carcinoma (HCC)
One of the earliest papers presenting evidence about the role of curcumin in prevention of HCC was published by Soni et al. in 1997 [108]. They showed that turmeric and curcumin inhibit the aflatoxin B1-induced mutagenesis in hepatic murine cells, reducing the genesis of gammaglutamyl-transpeptidase-positive foci. Furthermore, in 1998, Lin et al. [109] showed that curcumin decreases migration and invasion of a highly aggressive human HCC cell line through dose-dependent inhibition of MMP-9 secretion. In a N-diethylnitrosamine (DEN)-induced model of hepatocarcinogenesis conducted in C3H/NeH rats, Chuang et al. [110] found that administration of curcumin significantly decreases both incidence and multiplicity of HCC; similar results were achieved in subsequent studies conducted by different authors in female Wistar rats [111–113]: they all showed the protective role of curcumin in in vivo models of DEN-induced HCC. In these studies the suppressive effects of curcumin in the DENA-initiated alteration of hepatic foci in rats was accompanied by the restoration of normal levels of several hepatic metabolic enzymes. Busquets et al. [114] evaluated the effects of curcumin in a xenograft model of hepatocarcinogenesis; they demonstrated that the administration of curcumin can decrease the growth rate of HCC in Yoshida ascites hepatoma in rats, even with no influence on the tumor volume reduction, thus excluding a possible effect of curcumin on cell apoptosis. The effects of curcumin in combination with standard chemotherapeutic agents have also been investigated in vitro. In an interesting study, Notarbartolo et al. [115] showed that the combination of curcumin with cisplatin and doxorubicin results in synergistic cell growth inhibition in HA22T/VGH cells. Despite the reported positive evidence in some preclinical models, several studies have documented the failure of curcumin in protecting against chemical hepatocarcinogenesis in rodents. Curcumin failed to prevent HCC in rats treated with different chemical tumor inducers including N-ethyl-N-hydroxy-ethylnitrosamine, 3, 2’-methyl-4-aminobiphenyl [116] 3, 2’-methyl-4-aminobiphenyl, DENA/2-amino-3,8-dimethylimidazo[4,5-f] quinoxaline [117, 118], and 3,3’,4,4’-tetrachlo-robiphenyl [119]. Curcumin also failed to protect against copper-induced HCC in rats [120]. In the aforementioned studies, curcumin was administered in a relatively low dose, which probably prevented the occurrence of its pro-oxidants effects. In a very recent in vitro study, Cao et al. [121] evaluated the concomitant administration of sorafenib and curcumin; the authors assembled sorafenib and curcumin codelivered nanoparticles (SCN) and then measured their antiproliferative effects, in comparison with free sorafenib, curcumin and a physical mixture of sorafenib and curcumin (Sora + Cur). The coassembled SCN presented significantly enhanced antiproliferative effects compared to sorafenib, curcumin (6.0 and 1.5-fold higher reduction of tumor growth rate, respectively), and their physical mixture. In the same study, in vivo antiproliferative and antiangiogenic activities of SCN were determined by immunohistochemistry measurements. The captured images showed that the neovasculature was detected in the tumor sections from control, sorafenib, curcumin, and Sora + Cur groups, but was barely detected in the SCN-treated group, which implicated the higher antiangiogenic capability of SCN over other chemotherapy regimens. This evidence verified the enhanced therapeutic efficacy on in vivo antiproliferative and antiangiogenic activities by SCN.
Conclusions
There are numerous etiological factors able to elicit chronic liver inflammation that could lead to hepatic fibrosis and cirrhosis, including metabolic, infectious and autoimmune diseases. Despite the availability of etiologic-specific treatments for several liver diseases, no drugs or medications are currently approved for the regression of hepatic fibrosis and cirrhosis, which represent the final consequences of chronic liver injury. The interest in anti-fibrotic therapeutic or preventive agents has recently risen due to the availability of a new class of DAA against HCV. Whereas the clinical efficacy of these brand new medications has been widely demonstrated in eradicating HCV infection [2, 122–128], little is known about their effects on liver fibrosis and a significant histological improvement of cirrhosis with antiviral agents is probably unfeasible.
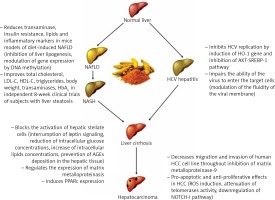
Curcumin is a natural compound easily extractable from turmeric and is widely used in middle-eastern diets. Several studies have uncovered its role in the modulation of many biological mechanisms involved in liver injury, and they are summarized in Figure 1.
The available data make curcumin a promising phytotherapy in chronic hepatitis and a potential therapeutic agent for regression of liver fibrosis and cirrhosis, especially in the setting of HCV infection, which could be easily eradicated with many direct-acting antivirals. Even though results from studies conducted so far are encouraging, several critical issues still must be overcome before curcumin may become available in clinical practice. The above-mentioned poor bioavailability of curcumin is a critical limiting factor which affects the drug pharmacodynamics, and then the use of a novel drug delivery system for curcumin must be considered when testing its clinical effects.