Introduction
Endometrial carcinoma (EC), an epithelial malignancy of the endometrium [1], accounts for 20% to 30% of female reproductive system tumors and ranks as the third most common malignancy of the female genital tract in developed countries [2]. Endometrial squamous cell carcinoma (ESC) is one of the subtypes of uterine tumors, and recent clinical studies have shown that there is an evident trend towards the younger population [3]. Although therapeutic strategies including a surgical operation that involves uterus removal [4], adjuvant therapy such as radiation therapy and chemotherapy [5] have generally been shown to improve the survival rate of early ESC, the prognosis of ESC remains unsatisfactory due to an extremely high rate of metastasis, cancer progression [6] and the occurrence of heart disease [7]. Therefore, the primary task of improving ESC prognosis is to study its pathogenesis to discover effective therapeutic targets for ESC treatment.
Approximately 2% of the human genome consists of protein-coding genes, whereas the rest is non-protein coding [8]. Studies later showed that the transcripts of “functionless genes” are non-coding RNAs (ncRNAs) with important biological and regulatory functions [9]. ncRNAs include miRNAs (micro RNA), tRNAs (transfer RNA), rRNAs (ribosomal RNA), asRNA (antisense RNA), snRNA (small nuclear RNA), as well as long-non coding RNAs (lncRNAs) which are more than 200 nucleotides in length [10, 11]. HOX genes, known as developmental genes, play a key role during embryogenesis [12]. Hundreds of ncRNAs have been discovered through gene chip detection of the HOX gene cluster transcripts [13]. A lncRNA, HOTAIR (HOX transcript antisense intergenic RNA), has been identified in the antisense strand of the HOXC gene cluster on chromosome 12 [14, 15]. HOTAIR is upregulated in a variety of tumors [16–20]. Subsequent studies found that the upregulation of HOTAIR is associated with the occurrence, development, metastasis and prognosis of tumors [19]. Dysregulation of HOTAIR expression significantly inhibits proliferation, invasion and migration of tumor cells [21, 22]. Interestingly, a previous study showed that decreased expression of HOTAIR enhances the sensitivity of cells to chemotherapy drugs [23]. Some studies have found upregulated expression of HOTAIR in endometrial cancer which is associated with poor prognosis [15, 24]. Although the roles of HOTAIR in the pathogenesis of ESC have been investigated in different studies, the underlying mechanism remains unclear.
miRNAs, a subtype of ncRNAs 21–23 nucleotides in length, induce mRNA degradation by binding to the 3’UTR of their target mRNAs [25]. It has been shown that miRNAs contribute to the progression of various human cancers [26, 27], and miRNAs act as potential tools or biomarkers for early cancer diagnosis, prognosis as well as treatment [28, 29]. MiR-152-3p is downregulated in various cancers and is known to be a typical tumor suppressor gene. miR-152-3p overexpression inhibits proliferation of cancer cells and increases the sensitivity of anti-cancer drugs [30–35]. DNA methylation has been shown to lead to the silencing of miR-152-3p in endometrial cancer, and the restoration of miR-152-3p inhibits the occurrence of endometrial cancer [36]. However, the functional roles of miR-152-3p in ESC and the underlying molecular mechanisms are still poorly understood.
LIN28B is the homologous gene of LIN28 in mammals and they share similar functions. LIN28B is highly expressed in embryonic stem cells and developmental tissues, whereas in mature tissues the expression of LIN28B is suppressed [37]. LIN28B promotes tumor initiation, development and metastasis through participating in the metabolic regulatory process of malignant tumors [38], and its expression is significantly upregulated in a variety of tumor tissues with high malignancy [39]. However, the role of LIN28B in ESC is still unclear.
In our present study, we aimed to investigate the effect of HOTAIR on the progression of ESC and to explore the mechanistic links between HOTAIR and ESC. The findings reported in this study are of clinical significance with the identification of miR-152-3p and LIN28B as the downstream regulators of HOTAIR in ESC. Our findings provide a basis for the development of predictive biomarkers for ESC.
Material and methods
Cell culture
Two human ESC cell lines (HEC-6 and ME-180) were used in this study. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL) with 10% (v/v) fetal bovine serum (FBS, HyClone), 100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen) in a 37°C constant temperature incubator with 5% CO2.
RNA preparation and qRT-PCR
RNA samples were extracted by the Trizol reagent (Invitrogen). cDNAs were synthesized using the Reverse Transcription System Bestar qPCR RT Kit according to the manufacturer’s instructions on an ABI 7500 Real-Time PCR System (Applied Biosystems). β-actin was used as the internal reference. Primer sequences are shown in Table I.
Table I
List of primers for qRT-PCR of different targets
Plasmid construction and transfection
HOTAIR-small interfering RNA (si-HOTAIR), miR-152-3p mimics were synthesized and their sequences are shown in Table II. PCR was used to amplify HOTAIR and LIN28B (primer sequences are listed in Table III). pcDNA3.1 expression plasmids (Invitrogen) were then constructed and sequenced. Plasmids were transfected into cells using Lipofectamine 2000 (Invitrogen, CA) according to the manufacturer’s protocols.
Target prediction and luciferase assay
Starbase (http://starbase.sysu.edu.cn) was used to predict binding sites for miR-152-3p in HOTAIR, and the putative targets of miR-152-3p were identified by TargetScan (http://www.targetscan.org). LIN28B wild-type and mutant 3′UTR reporter vectors were constructed and subcloned into the pGL3 basic firefly luciferase reporter. For the dual luciferase activity, LIN28B-wt or its mutant counterpart LIN28B-mut was cloned into the 3′UTR of the Renilla luciferase gene in pRL-TK (Promega, USA). Each plasmid was co-transfected along with miR-152-3p mimics or negative control mimics into cells. The firefly luciferase gene in the pGL3 control was used as a control for transfection efficiency. The firefly luciferase and Renilla luciferase signals were detected using the dual-luciferase assay reporter kit and Lumat LB 9501 luminator.
RNA pull-down
RNA pull-down was performed to detect the aggregation of miR-152-3p using the qRT-PCR biotin-labeled HOTAIR as a probe. DNA fragments containing the miRNA-152-3p binding site in HOTAIR were cloned into pCR8 (Invitrogen) as previously described [40]. Plasmids were then transfected into HEC-6 and ME-180. A total of 2 μg of cell lysates was mixed with biotinylated RNA, and then incubated with streptavidin agarose beads at room temperature for 1 h. The co-precipitated RNAs were purified using the TRIZOL reagent and detected by qRT-PCR.
Western blot
Cells were collected and snap frozen in liquid nitrogen. Cells were lysed using the ultrasonic cell-break method and incubated in 50 mM lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM PMSF, 10 mM β-glycerophosphate, 1% Triton X-100, 5 mM EDTA, 0.2 mM Na3VO4, 2 µg/ml leupeptin, and 2 µg/ml pepstatin A) on ice. Cell homogenates were centrifuged at 12 000 × g at 4°C for 30 min, and the supernatant was collected. Protein lysates (30 μg) were loaded onto sodium dodecyl sulfate-polyacrylamide gels for electrophoresis and transferred to PVDF membranes. Membranes were blocked with PBS-T containing 5% BSA, followed by incubation overnight at 4°C with the primary antibody as follows: monoclonal antibodies (1 : 1000 dilution in PBS-T, Santa Cruz, CA, USA) against MMP2, MMP9, LIN28B. Then, membranes were washed with TBST (10 min for 3 times), and then probed with the appropriate secondary antibody (1 : 5000; Abcam). Immunoreactivity was determined and observed using enhanced chemiluminescence (Millipore, USA). GAPDH was used as a control.
Cell invasion and migration
5 × 104 HEC-6 and ME-180 cells were seeded into each well of the upper chamber (BD BioCoat Matrigel Invasion Chamber; BD Biosciences, USA) 48 h after transfection. Non-invasive cells were removed by a cotton swab. The invasive cells were fixed in methanol, washed with PBS and stained with DAPI. Next, cells were counted and photographed using a microscope (× 100) in at least three independent experiments.
Scratch wound assay was used to evaluate migration of ESC cells. 1 × 105 transfected HEC-6 and ME-180 cells were seeded into each well in a 6-well plate. A 200 μl pipette tip was used to gently scratch the cell monolayer to form a wound gap, and wells were washed with PBS three times. 10% FBS-supplemented DMEM was then added and cells were maintained for an additional 24 h. At time points 0 h and 24 h, cells were photographed using an inverted microscope to record the wound width.
Statistical analysis
All data are presented as mean ± SEM. All experiments were performed at least three independent times. The difference between groups was analyzed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple-comparison test using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was regarded as statistically significant.
Results
HOTAIR promotes migration and invasion of ESC
In order to detect the functional role of HOTAIR in ESC, we constructed plasmids that highly expressed the HOTAIR gene (HOTAIR), whereas HOTAIR knockdown was achieved by using siRNA (siHOTAIR). The efficiencies of HOTAIR plasmid and siHOTAIR transfection were validated by qRT-PCR, which showed a significant increase and reduction in HOTAIR expression, respectively, in both HEC-6 and ME-180 (p < 0.001) (Figure 1 A). Data from cell scratch (Figure 1 B) and transwell migration assays (Figure 1 C) of the HOTAIR group demonstrated that cell migratory and invasive ability was dramatically increased compared with the control group (p < 0.001), while the siHOTAIR-treated group showed decreased cell migration (p < 0.001) (Figures 1 D and E) and invasion (p < 0.001) (Figure 1 E). Previous studies have reported the roles of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) in cleaving matrix proteins, and the association of MMP proteins with cancer cell invasion and metastasis [41, 42]. In this study, MMP-2 and MMP-9 were significantly increased in the HOTAIR group and decreased in the siHOTAIR group in both HEC-6 and ME-180 (Figure 1 F), suggesting that HOTAIR overexpression may promote migration and invasion of ESC.
Figure 1
HOTAIR promotes migration and invasion of endometrial squamous carcinoma (ESC). A – Transfection efficiencies of HOTAIR overexpression and siHOTAIR were determined by qRT-PCR. B – Effect of HOTAIR on the migratory ability of HEC-6 and ME-180 C – Effect of HOTAIR on the migratory and invasive ability of HEC-6 and ME-180. D – Effect of siHOTAIR on the migratory ability of HEC-6 and ME-180 E – Effect of siHOTAIR on the migratory and invasive ability of HEC-6 and ME-180. F – Western blot analysis shows a significant increase and decrease in MMP-2 and MMP-9 expression in HOTAIR- and siHOTAIR-transfected HEC-6 and ME-180, respectively
***p < 0.001; si-HOTAIR – HOTAIR-small interfering RNA, qRT-PCR – quantitative real-time polymerase chain reaction.

miR-152-3p as a target of HOTAIR
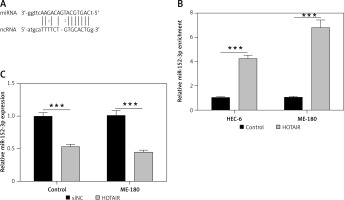
Next, we used the computational prediction function in Starbase to identify potential binding sites for miR-152-3p in HOTAIR (Figure 2 A). The binding ability between miR-152-3p and HOTAIR was confirmed by RNA pull-down assay. Cells transfected with HOTAIR plasmid showed increased expression of miR-152-3p as captured by RNA pull-down assay, supporting the binding of miR-152-3p to HOTAIR (p < 0.001) (Figure 2 B). The expression of miR-152-3p was significantly decreased in ESC cells (p < 0.001) (Figure 2 C), suggesting that increased HOTAIR in ESC could inhibit the expression of miR-152-3p.
HOTAIR promotes migration and invasion of ESC by inhibiting miR-152-3p
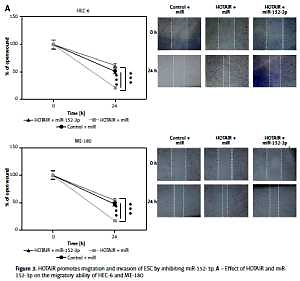
To further assess whether HOTAIR promotes cell migration and invasion by specifically targeting miR-152-3p, we examined three groups of HEC-6 and ME-180 models: control + miR group, HOTAIR + miR group (overexpression of HOTAIR) and HOTAIR + miR-152-3p (overexpression of HOTAIR and miR-152-3p) group. Cell scratch (Figure 3 A) and transwell migration assays (Figure 3 B) demonstrated that cell invasion and migration were significantly increased in the HOTAIR + miR group compared with the other two groups with similar efficiencies (p < 0.001), suggesting that HOTAIR promotes migration and invasion of ESC by inhibiting miR-152-3p. The expression of MMP-2 and MMP-9 was also significantly increased in the HOTAIR + miR group compared with the other two groups with similar efficiencies (Figure 3 C), which provides further evidence supporting the above findings.
Figure 3
HOTAIR promotes migration and invasion of ESC by inhibiting miR-152-3p. A – Effect of HOTAIR and miR-152-3p on the migratory ability of HEC-6 and ME-180 B – Effect of HOTAIR and miR-152-3p on the migratory and invasive ability of HEC-6 and ME-180. C – Western blot analysis shows a significant increase in MMP-2 and MMP-9 expression in HOTAIR-transfected HEC-6 and ME-180, and restoration of MMP-2 and MMP-9 to normal levels in the HOTAIR + miR-152-3p group
***p < 0.001.
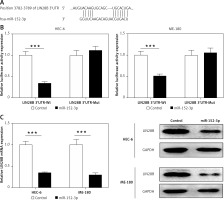
LIN28B as a target of miR-152-3p
We used a computational prediction program, TargetScan, to identify the potential binding sites for LIN28B mRNA in miR-152-3p. The 3′UTR of LIN28B mRNA was found to bear the miR-152-3p binding site (Figure 4 A). Regulation of the LIN28B expression by miR-152-3p was evaluated by luciferase reporter assays. Overexpression of miR-152-3p significantly decreased luciferase activity of the reporter gene with wild-type LIN28B 3′UTR compared with the negative control (p < 0.001) (Figure 4 B). However, the inhibitory effect of miR-152-3p on LIN28B expression was rescued when the predicted miR-539-binding site in 3′UTR of LIN28B mRNA was mutated (Figure 4 B). qRT-PCR and western blot analysis further confirmed that the mRNA and protein levels of LIN28B were downregulated by overexpression of miR-152-3p (p < 0.001) (Figure 4 C). The above results showed that miR-152-3p inhibited expression of LIN28B by binding to LIN28B 3′UTR.
Figure 4
LIN28B as a target of miR-152-3p. A – Predicted binding sites for miR-152-3p in the 3′UTR of LIN28B and mutations in the binding sites are shown. B – Luciferase reporter assay of LIN28B 3′UTR-wild type and mutant in HEC-6 and ME-180. C – qRT-PCR and western blot analysis of LIN28B in HEC-6 and ME-180 transfected with miR-152-3p plasmid
***p < 0.001.
miR-152-3p inhibits cell migration and invasion by targeting LIN28B
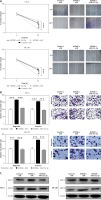
In order to evaluate the functional roles of miR-152-3p in ESC, the miR-152-3p overexpression plasmid (miR-152-3p) was constructed. A significant increase in miR-152-3p expression confirmed the transfection efficiency in HEC-6 and ME-180 (p < 0.001) (Figure 5 A). Data from cell scratch (Figure 5 B) and transwell migration assays (Figure 5 C) demonstrated that cell migratory and invasive ability was dramatically decreased in the miR-152-3p group compared with the control group (p < 0.001), suggesting the inhibitory role of miR-152-3p in ESC progression. Next, we examined three groups of HEC-6 and ME-180 models: miR + control group, miR-152-3p + control group (overexpression of miR-152-3p) and miR-152-3p + LIN28B (overexpression of miR-152-3p and LIN28B) group. Transwell migration (Figure 5 D) and cell scratch (Figure 5 E) assays demonstrated that cell invasion and migration were significantly decreased in the miR-152-3p + control group compared with the other two groups with similar efficiencies (p < 0.001). Overexpression of LIN28B in the miR-152-3p + LIN28B group restored cell migration and invasion. The expression of MMP-2 and MMP-9 was significantly decreased in the miR-152-3p group (Figure 5 F), confirming the ability of miR-152-3p in inhibiting ESC progression. Furthermore, the protein levels of LIN28B in the miR-152-3p + control group in both HEC-6 and ME-180 were significantly decreased compared to the miR + control and miR-152-3p + LIN28B groups (Figure 5 G), indicating the inhibition of LIN28B expression by miR-152-3p. These results suggest that miR-152-3p inhibits migration and invasion of ESC by targeting LIN28B. The expression of MMP-2 and MMP-9 in both HEC-6 and ME-180 was also significantly decreased in the miR-152-3p + control group compared with the other two groups (Figure 5 H), which provides further evidence supporting the above findings.
Figure 5
miR-152-3p inhibits cell migration and invasion by targeting LIN28B. A – Transfection efficiency of miR-152-3p overexpression in HEC-6 and ME-180 as determined by qRT-PCR. B – Effect of miR-152-3p on the migratory ability of HEC-6 and ME-180 C –Effect of miR-152-3p on the migratory and invasive ability of HEC-6 and ME-180. D – Effect of miR-152-3p and LIN28B on the migratory and invasive ability of HEC-6 and ME-180 E – Effect of miR-152-3p and LIN28B on the migratory ability of HEC-6 and ME-180. F – Western blot analysis shows a significant decrease in MMP-2 and MMP-9 in HEC-6 and ME-180 transfected with miR-152-3p plasmid G – Western blot analysis of LIN28B in miR + control group, miR-152-3p + control group and miR-152-3p + LIN28B group of HEC-6 and ME-180, show a decrease in LIN28B in miR-152-3p + control compared to other two groups. H – Western blot analysis shows a significant decrease in MMP-2 and MMP-9 in HEC-6 and ME-180 transfected with miR-152-3p plasmid alone. MMP-2 and MMP-2 expression is restored to normal levels in HEC-6 and ME-180 transfected with miR-152-3p + LIN28B
***p < 0.001.

Discussion
In this study, we demonstrated the ability of HOTAIR to promote ESC and revealed the potential mechanism. Specifically, we showed that overexpression of HOTAIR promoted migration and invasion of ESC using transwell migration and cell scratch assays. Moreover, knockdown of HOTAIR using in vitro human ESC cell line models suppressed tumor migration and invasion. We identified miR-152-3p as a novel target of HOTAIR using RNA pull-down. We also confirmed that HOTAIR inhibited the expression of miR-152-3p. By using a dual luciferase reporter assay, we demonstrated that miR-152-3p could target tumor promoter LIN28B and inhibited the expression of LIN28B. Finally, we showed that HOTAIR suppressed miR-152-3p and promoted LIN28B, leading to ESC progression.
Studies have indicated the association of aberrant lncRNA expression with cancers and the crucial role played by lncRNAs in the modulation of cancer development and progression [43], suggesting that lncRNAs may act as potential biomarkers and therapeutic targets for cancers. Therefore, the identification of lncRNAs is critical in developing advanced treatment strategies for ESC. At present, only a few lncRNAs related to ESC have been identified [15]. Studies have shown that HOTAIR acts as a cancer gene that promotes migration and invasion of various tumors, such as breast cancer [20] and hepatocellular carcinoma [44]. However, the specific mechanism of how HOTAIR promotes tumor progression remains unclear. Liu et al. [45] reported that HOTAIR could modulate p21 expression to promote lung adenocarcinoma progression. HOTAIR is known to be upregulated in ESC cells and tumor tissues of ESC patients, and is associated with poor prognosis of patients [24]. More importantly, abnormal expression of HOTAIR is positively correlated with TGF-β1 expression, indicating the association of HOTAIR with the occurrence and development of EC [22, 46]. However, little is known about the functions of HOTAIR in ESC. In this study, we found that HOTAIR was highly expressed in HEC-6 and ME-180 and could play a carcinogenetic role in ESC by inducing cell migration and invasion. Moreover, HOTAIR has been found to regulate VEGF, MMP-2 and MMP-9 in a wide variety of tumor cells, enhances the tumor biological behavior, and promotes tumor metastasis. Suppression of HOTAIR inhibits cell proliferation and reduces MMP protein expression [47]. Our western blot results showed that overexpression of HOTAIR increased MMP-2 and MMP-9 expression in ESC cells, while HOTAIR knockdown resulted in significant reduction in MMP-2 and MMP-9 expression. Taken together, these findings revealed the fundamental role of HOTAIR in ESC progression.
LncRNAs act as sponges for miRNAs to regulate the expression of target genes [48] and are therefore known as competing endogenous RNAs (ceRNAs). For example, HOTAIR acts as a ceRNA to promote malignant melanoma progression by sponging miR-152-3p [49]. Through negatively regulating miR-130a, HOTAIR acts as a driver of malignancy in gallbladder cancer [50]. These studies revealed the functional role of HOTAIR in tumor progression via regulation of downstream miRNAs or protein-coding genes. It has been found that the restoration of miR-152-3p could inhibit the occurrence of endometrial cancer [36]. Similarly, a reduced expression level of miR-152-3p was found in ESC, suggesting that miR-152-3p may have a functional role in the progression of ESC. In our study, we revealed that HOTAIR promoted ESC progression by targeting and inhibiting miR-152-3p. Our findings also suggest that miR-152-3p plays a vital regulatory role in ESC progression, and may have protective effects against ESC development and metastasis. Thus, miR-152-3p should be considered as a potential therapeutic target for ESC.
Furthermore, we identified the potential binding sites for miR-152-3p in LIN28B by using TargetScan. LIN28B is known to be a suppressor of miRNA biogenesis, which is implicated in progression and metastasis of endometrial carcinosarcomas [51]. Moreover, increased LIN28B expression is correlated with reduced expression of let-7b and overexpression of HMGA2 [52, 53], thereby stimulating epithelial-to-mesenchymal transition and EC progression. These findings suggest the possible role of HOTAIR in the upregulation of LIN28B in ESC. In our study, luciferase reporter assay, qRT-PCR and western blot analysis showed that miR-152-3p inhibited the expression of LIN28B by binding to the 3′UTR of LIN28B. Moreover, miR-152-3p inhibited migration and invasion of ESC by targeting LIN28B. These findings provided a mechanistic insight into the role of miR-152-3p as well as the novel regulatory role of LIN28B in ESC. However, future studies are required to investigate the molecular mechanism in vivo in ESC patients and to evaluate the role of the HOTAIR/miR-152-3p/LIN28B axis in a clinical context.
In conclusion, to the best of our knowledge, this is the first study demonstrating the role of HOTAIR in promoting tumor migration and invasion in ESC by specifically targeting miR-152-3p/LIN28B. In conclusion, we showed that HOTAIR is an oncogene in ESC with a binding site for miR-152-3. We confirmed and provided new insights into the role of HOTAIR in promoting ESC metastasis by targeting miR-152-3p, thereby functionally activating the downstream LIN28B. Understanding the regulatory mechanism of HOTAIR in ESC could lead to the identification of useful clinical biomarkers or indicators. In addition, miR-152-3p provides efficient protective effects against the progression of ESC, suggesting that miR-152-3p may be a potential therapeutic target for developing novel ESC treatment.