Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract [1]. Patients with inflammatory bowel disease suffer from malabsorption and malnutrition and therefore may be at risk of polyunsaturated fatty acid deficiency. There are some indications that patients with inflammatory bowel disease are at risk of essential fatty acid insufficiency (EFAI) and, in severe cases, of essential fatty acid deficiency (EFAD) [2]. The human body produces both saturated fatty acids and unsaturated fatty acids, but two fatty acids – linoleic acid [C18:2(n-6)] and α-linolenic acid [C18:3(n-3)] – cannot be produced in the body. When sufficient amounts of α-linolenic acid and linoleic acid are present in the body, other essential fatty acids (EFA) – n-3 and n-6 fatty acids – can be synthesized. α-Linolenic acid is metabolized into eicosapentaenoic acid (EPA) [C20:5(n–3)] and later into docosahexaenoic acid (DHA) [C22:6(n–3]). Linoleic acid is transformed into arachidonic acid [C20:4(n–6)] [3]. Patients who develop severe EFAD have dramatic symptoms, such as substantial hair loss and overt clinical dermatitis. Biochemical evidence of EFAD, such as the ratio 20:3(n-9)/20:4(n-6) > 0.4, can be detected in patients. EFAI is characterized by an imbalance of the polyunsaturated FA (n-3)/polyunsaturated FA (n-6) ratio. The ratio of 16:1(n-7)/18:2(n-6), 20:3(n-9)/20:4(n-6) and PUFAs/noPUFAs is used to monitor EFA status [2]. PUFA depletion is related to disease activity. Fatty acids play an important biological role in the regulation of membrane structure and function, regulation of intracellular signaling pathways, and regulation of the production of bioactive lipid mediators, especially modifying the response to a number of metabolic processes including inflammation.
Diet modification is one of the recommended approaches to treating IBD patients. The use of an anti-inflammatory diet (AID) that restricts the intake of carbohydrates with the addition of appropriate fatty acids is advised [4]. Levine et al. [5] provide guidance which has been compiled by the nutrition cluster of the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) regarding food groups, food additives, specific dietary components which may be beneficial and safe to consume for patients with inflammatory bowel diseases. They held off on endorsing the use of n-3 supplements [5]. In contrast, for patients with IBD Scaioli et al. [6] recommended supplementation with high-purity n-3 preparations, which have an anti-inflammatory effect and may be helpful in preventing clinical relapse in patients with ulcerative colitis.
There are no data regarding the phospholipid fatty acid profile both in serum and in a colon biopsy specimen in patients with inflammatory bowel diseases. The aim of the study was to address the fatty acid status and its relationship with disease activity.
Methods
The study included 17 patients (9/8, F/M) with IBD. Ten patients without abnormality confirmed during colonoscopy examination (6/4, F/M) served as controls. The mean age (± SD) of IBD patients was 47.5 ±17.6 years, and 67.5 ±11.7 years in the control group (p = 0.014). None of the patients was treated with specific nutritional interventions which might influence fatty acid status. The exclusion criteria were as follows: malignancy, diabetes mellitus, obesity (body mass index ≥ 30 kg/m2), cardiovascular disease, body mass index < 18.5 kg/m2. All subjects (patients with IBD and controls) have had colonoscopy examination in the Outpatient Gastroenterology and Hepatology Clinic at Jagiellonian University Medical College in Krakow, Poland. Before the colonoscopy procedure, fasting venous blood samples were taken from patients. A colon biopsy specimen involved and an adjacent colon biopsy specimen not involved in the inflammatory process were taken from patients during the colonoscopy procedure. The severity of active histological inflammation was assessed using the Geboes score [7].
Measurement of the level of individual fatty acids of the phospholipid fraction in the serum and colon biopsy specimen was performed using gas chromatography with a flame ionization detector (Agilent Technologies 6890 Network GC System, Wilmington, De., USA) in the Department of Clinical Biochemistry, Institute of Pediatrics at Jagiellonian University Medical College in Krakow, Poland [8]. Usually, the results of fatty acids of tissue are expressed as the percentage of total fatty acids, and in the present study, for better comparability of results between serum and the colon biopsy specimen, the results were expressed as the percentage of total fatty acids in phospholipid fraction.
Statistical analysis
Descriptive statistics (mean values, SD, medians, quartiles Q1-Q3) were used in the statistical assessment of the results. Statistica software version 13 (StatSoft) was used to perform statistical analysis. To evaluate the distribution of continuous variables in terms of their compliance with the normal distribution, the Shapiro-Wilk test was employed. Student’s t-test was applied to compare the mean value of fatty acids for normally distributed continuous variables. In the case of non-normal distribution, the Mann-Whitney U test was used. Pearson’s correlation was used to examine relationships between the Geboes activity index and the level of fatty acids. A p-value less than 0.05 was considered statistically significant.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Jagiellonian University Ethics Committee (decision no. KBET/35/B/2014).
Results
In serum of patients with IBD significantly lower mean values of percentage of total fatty acid content of C18:3(n-3), C20:2(n-6) and the sum of PUFAs (p = 0.016; p = 0.034; p = 0.034, respectively) as compared to controls were found. In contrast, the mean value of C16 and the sum of SFAs were significantly higher in IBD patients as compared to controls (p = 0.023; p = 0.004, respectively) (Table I).
Table I
Mean (±SD) or median (interquartile range) values of serum/colon biopsy specimen fatty acids of phospholipid fraction in the control group and in patients with IBD, expressed as the percentage of total fatty acid content
| Fatty acid | Serum | Colon biopsy specimen | |||
|---|---|---|---|---|---|
| Controls | Study group | Controls | Macroscopically not involved in the disease process | Macroscopically involved in the disease process | |
| Percentage of total FAs [% FAs] Mean ± SD or median (interquartile range) | |||||
| SFAs: | 45.7 ±1.9 | 47.7 ±1.7 p = 0.004* | 50.8 ±4.4 | 68.2 ±8.8 p = 0.002* | 63.0 ±9.3 p = 0.006* |
| C 12 | 0.06 ±0.03 | 0.06 ±0.02 | 1.3 (0.8–2.4) | 0.44 (0.15–1.05) p = 0.002* | 0.28 (0.20–0.42) p = 0.0009* |
| C 14 | 0.55 ±0.18 | 0.45±0.11 | 2.1 (2.0–2.3) | 2.0 (1.8–3.2) | 1.6 (1.4–1.9) |
| C 16 | 32.5 (30.3–34.2) | 35.0 (33.9–35.2) p = 0.023* | 33.3 (31.2–36.1) | 40.5 (36.6–42.8) p = 0.047* | 37.2 (33.3–42.5) |
| C 18 | 11.6 ±1.4 | 11.7 ±1.0 | 15.4 ±2.0 | 26.4 ±5.7 p = 0.0008* | 23.1 ±5.2 p = 0.002* |
| C 24 | 0.90 ±0.23 | 0.95 ±0.26 | 0.45 ±0.15 | 0.39 ±0.1 | 0.42 ±0.14 |
| MUFAs: | 9.1 ±2.5 | 9.4 ±1.8 | 22.0 ±4.9 | 14.9 ±4.8 p = 0.05* | 15.6 ±5.1 p = 0.02* |
| C 16:1 (n-7) | 0.51 ±0.25 | 0.55 ±0.14 | 0.53 (0.42–0.93) | 0.67 (0.48–0.92) | 0.76 (0.56–0.86) |
| C 18:1 (n-9) | 8.6 ±2.5 | 8.9 ±1.7 | 21.2 ±4.7 | 14.3 ±4.6 p = 0.05* | 14.9 ±5.0 p = 0.017* |
| PUFAs: | 44.4 ±3.3 | 42.0 ±2.6 p = 0.034* | 24.7 ±3.1 | 15.0 ±5.0 p = 0.02* | 19.9 ±7.0 |
| C 18:2 (n–6) | 16.4 ±3.8 | 15.1 ±2.3 | 9.3 ±2.4 | 5.2 ±1.2 p = 0.003 | 5.8 ±1.9 p = 0.001 |
| C 18:3 (n–3) | 0.28 (0.16–0.39) | 0.13 (0.10–0.14) p = 0.016* | 0.31 (0.20–0.56) | 0.18 (0.15–0.21) | 0.16 (0.09–0.18) p = 0.002* |
| C 20:2 (n–6) | 0.59 ±0.15 | 0.46 ±0.15 p = 0.034* | 0.55 (0.48–0.61) | 0.34 (0.29–0.48) | 0.48 (0.39–0.69) |
| C 20:4 (n–6) | 16.8 ±3.2 | 16.1 ±3.9 | 11.3 ±2.3 | 7.2 ±3.4 | 10.5 ±4.5 |
| C 20:5 (n–3) | 1.6 ±0.9 | 1.1 ±0.4 | 0.94 ±0.37 | 0.43 ±0.07 p = 0.002* | 0.48±0.19 p = 0.0005* |
| C 22:6 (n–3) | 8.6 ±2.8 | 9.1 ±2.9 | 2.3 ±0.9 | 1.6 ±0.9 | 2.4 ±1.2 |
No significant difference between the mean or median values of each individual fatty acid measured in mucosal tissues involved in the disease process and in mucosal tissues not involved in the disease process was noted (Table I). Because of that, for comparison with controls, percentages of FAs from all mucosal tissues of patients with IBD were taken together for further analysis. The mean values of C16 and C18 percentage of total FAs, as well as the sum of SFAs in mucosal tissues of IBD patients, were significantly higher as compared to controls (p = 0.0029–0.000025). In contrast, the mean percentage values of C12 and monounsaturated acid (C18:1 (n-9)) and the polyunsaturated acids C18:2(n-6), C18:3(n-3), C20:5(n-3) were significantly lower in IBD patients as compared to controls (p = 0.00085–0.000004).
Significant negative correlations between the histological activity of inflammation of the disease assessed by the Geboes activity index and the ratios of PUFAs/noPUFAs, n-3 FAs and n-6 FAs were observed (r = –0.433, p < 0.01; r = –0.364, p < 0.04; r = –0.417, p < 0.02, respectively). In contrast, significant positive correlations between Geboes activity index and ratio of C16:1(n-7)/C18:2(n-6) and SFAs were found (r = 0.628, p < 0.0001; r = 0.436, p < 0.01, respectively).
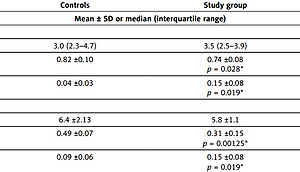
Among the calculated indices, the mean values of the ratio of PUFAs/noPUFAs were significantly lower in both the serum and colon mucosa in patients with IBDs as compared to controls (p = 0.028; p = 0.00125, respectively) (Table II).
Table II
Ratios of ∑ n-6/∑ n-3, PUFAs/noPUFAs, C16:1 (n-7)/C18:2 (n-6) in controls and in patients with IBD
| Indices | Controls | Study group |
|---|---|---|
| Mean ± SD or median (interquartile range) | ||
| Serum: | ||
| ∑ n-6/∑ n-3 | 3.0 (2.3–4.7) | 3.5 (2.5–3.9) |
| PUFAs/noPUFAs | 0.82 ±0.10 | 0.74 ±0.08 p = 0.028* |
| C16:1 (n-7)/C18:2 (n-6) | 0.04 ±0.03 | 0.15 ±0.08 p = 0.019* |
| Colon biopsy specimen: | ||
| ∑ n-6/∑ n-3 | 6.4 ±2.13 | 5.8 ±1.1 |
| PUFAs/noPUFAs | 0.49 ±0.07 | 0.31 ±0.15 p = 0.00125* |
| C16:1 (n-7)/C18:2 (n-6) | 0.09 ±0.06 | 0.15 ±0.08 p = 0.019* |
* vs. controls; ∑ n-6 = C18:2 (n-6) + C20:4 (n-6) + C20:2(n–6); ∑ n-3 = 18:3 (n-3) + C20:5 (n-3) + C22:6 (n-3); SFAs (saturated fatty acids) = C12 + C14 + C16 + C18 + C24; MUFAs (monounsaturated fatty acids) = C16:1(n-7) + C18:1 (n-9); PUFAs (polyunsaturated fatty acids) = C18:2(n-6) + C18:3(n-3) + C20:2(n-6) + C20:4(n-6) + C20:5(n-3) + C22:6(n-3); noPUFAs = SFA + MUFA.
Discussion
MUFA and PUFA contents in the serum phospholipid fraction reflect, to some extent, the dietary intake of fatty acids. Because of that, the measurement of FAs in the phospholipid fraction, both in serum and in mucosal tissue, seemed to be more appropriate when the role of fatty acid in IBD is studied [9].
It is known, that C18:1 is involved in mediating anti-inflammatory responses with immune cells [10]. In our study, the mean level of colon mucosa phospholipid C18:1 was significantly lower in patients with inflammatory bowel diseases than in controls. It may indicate the involvement of oleic acid in mediating anti-inflammatory responses in patients with IBD. It has already been confirmed in animals. The fatty acid composition of acorn-fed ham, with very high levels of the anti-inflammatory oleic acid and a low n-6/n-3 ratio, may serve as a prevention strategy for ulcerative colitis onset or progression, as has been demonstrated in an animal model. This diet induced changes in gut microbiota composition, with pronounced enrichments in anti-inflammatory bacterial genera [11]. Additionally, it is known, that probiotics (live microorganisms) have a beneficial effect on health by exhibiting quantitative and qualitative effects on intestinal microflora and/or modification of the immune system [11, 12].
In the present study, the mean serum/colon mucosa level of SFAs was significantly higher in patients with inflammatory bowel diseases than in controls, with the greatest contribution of C16. It should be stressed that mucosa SFA levels were positively correlated with disease activity in patients with IBD. In the most recent studies, the role of C16 in mediating the function of isolated macrophages and the pro-inflammatory effects on macrophages in conditions such as obesity or when high-fat diets are ingested have been indicated [10].
It is suggested that C18:3(n-3) could ameliorate the inflammatory damage in colitis [13]. In the present study, the level of phospholipid C18:3(n-3), both in serum and colon mucosa, was significantly lower in patients with inflammatory bowel diseases than in controls. Additionally, the mean level of colon mucosa phospholipid C20:5(n-3) was significantly lower in patients with inflammatory bowel diseases than in controls. The results are in agreement with the study done by Uchiyama et al. [14], who obtained a lower level of EPA from the phospholipid fraction of the erythrocyte membrane in patients with IBD than in the healthy subjects. This diminished level of C18:3 clearly confirmed the deficiency of n-3 polyunsaturated fatty acids in patients with IBD.
Most of the studies have focused on C18:2(n-6) and C20:4(n-6) as eicosanoid precursors, whereas C18:2(n-6) is one of the most important essential fatty acids. Most reports of EFAD showed C18:2(n-6) deficiency, with little comment of C18:3 (n-3) deficiency [15]. Jeppesen et al. [16] found that patients with higher degrees of malabsorption had lower C18:2(n-6) levels. In the present study, the level of phospholipid C18:2(n-6), both in colon mucosa macroscopically not involved in the disease process and colon mucosa macroscopically involved in the disease process, was significantly lower than in controls. Additionally, the levels of PUFAs (n-6) and PUFAs (n-3) decreased with increasing histological activity of inflammation of the disease assessed by the Geboes activity index.
In IBD patients with intestinal fat malabsorption and suspected EFA deficiency, decreased concentrations of 18:2(n-6) and ratios of essential fatty acids to nonessential fatty acids were found [17]. The ratio of PUFAs/noPUFAs and the ratio of 16:1(n-7) to 18:2(n-6) are used to measure EFAI/EFAD [2]. A decrease of PUFA/noPUFA ratio both in the serum and colon mucosa in patients with IBD was noted in the present study, which is in agreement with the results obtained by Siguel [2]. Moreover, inverse significant correlations between PUFAs/noPUFAs and the Geboes activity index as well as C16:1/C18:2 and the Geboes activity index in tissues were noted. Decreased PUFAs and increased SFAs are associated with the severity of the intestinal disease. The insufficiency of fatty acids was so deep that the profile of fatty acids had changed not only in serum but also in colon mucosa macroscopically not involved in the disease process and colon mucosa macroscopically involved in the disease process.
There are two limitations of our study. The first is the significant difference in age between controls and IBD patients. However, it has to be taken into account that in controls, IBD was excluded and no inflammation was present in the intestine. So, the age difference between the study group and controls seem not to be the cause of differences in fatty acid status. The second is the small number of patients. However, the results of the study clearly show the differences between inflamed tissue of IBD patients and the tissue not changed by the disease process. Although the study has some limitations, it is tempting for clinicians to advise PUFA supplementation in IBD patients. A large, well-designed and adequately powered clinical trial is necessary to assess the influence of fatty acid supplements for treating patients with IBD.
In conclusion, the fatty acid profile of phospholipids in serum and in colon biopsy specimen in patients with IBD is characteristic for essential fatty acids insufficiency. It may indicate, that fatty acid supplementation could be advantageous in patients with inflammatory bowel disease.