Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting millions of people worldwide and posing a significant public health burden due to its association with increased risk of stroke, heart failure, and mortality [1, 2]. Observational studies have consistently suggested a potential association between blood pressure parameters, including systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP), and the development of AF [3, 4]. However, these observational associations may be confounded by various environmental and lifestyle factors, making it challenging to establish causality.
Importantly, growing evidence suggests that there might be sex-specific differences in the pathophysiology and risk factors of AF. Previous epidemiological studies have reported varying prevalence, incidence, and outcomes of AF between males and females, with females often showing different risk factor profiles and worse prognosis [5–7]. However, whether the relationship between blood pressure and AF risk differs between sexes remains poorly understood, and the causal nature of these sex-specific associations has not been well established.
To address these knowledge gaps, we employed a sex-stratified two-sample Mendelian randomization (MR) approach. MR utilizes genetic variants as instrumental variables to investigate causal relationships while minimizing confounding and reverse causation [8]. By analyzing both sex-pooled and sex-specific genetic data, our study aimed to elucidate the potential causal relationships between different blood pressure parameters (SBP, DBP, and PP) and AF risk, with a particular focus on identifying any sex-specific patterns in these associations.
The GWAS summary data of blood pressure phenotypes (including sex-pooled SBP, female-specific SBP, male-specific SBP, sex-pooled DBP, female-specific DBP, male-specific DBP, sex-pooled PP, female-specific PP and male-specific PP) were obtained from a recent study based on sex-specific genetic architecture of blood pressure. Detailed information, such as recruitment criteria of population and quality control of genetic data, can be found in the original paper [9]. The GWAS summary data of sex-pooled AF, female-specific AF, and male-specific AF were obtained from Neale Lab UKBB GWAS round 2. Detailed information, such as recruitment criteria of population and quality control of genetic data, can be found on the website https://www.nealelab.is/uk-biobank.
Out study carefully selected genetic instruments following stringent criteria to ensure robust causal inference. Single nucleotide polymorphisms (SNPs) were selected as instrumental variables based on their association with blood pressure phenotypes using genome-wide significant thresholds (p < 5 × 10–8). To minimize potential bias from linkage disequilibrium (LD), we performed LD pruning with a threshold of r2 < 0.001, ensuring the independence of selected genetic instruments. SNPs with a minor allele frequency (MAF) of ≤ 0.01 were excluded to mitigate potential bias.
The study employed five analytical methods including MR-Egger, weighted median, inverse-variance weighted (IVW), simple mode, and weighted mode. The IVW method served as the primary methodology. The results, after correction for false discovery rate (FDR) with a threshold of p < 0.05, were considered to indicate a causal relationship between blood pressure phenotypes and the risk of AF.
MR-Egger and IVW methods were employed to assess the heterogeneity of the analysis results. A result with Q_pval < 0.05 was considered to indicate the presence of heterogeneity. The MR-Egger method was also employed to assess the pleiotropy of the analysis results. A result with p-value < 0.05 was considered to indicate the presence of pleiotropy.
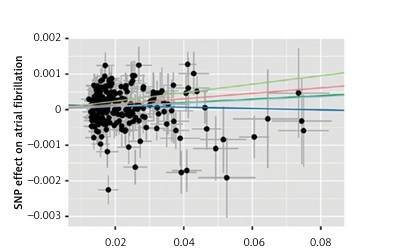
Instrumental variables were selected from GWAS datasets for blood pressure phenotypes based on the criteria established in this study. Detailed information on all IVs, including SNP rsIDs, effect sizes (beta coefficients), alleles (effect and other), standard errors (SEs), effect allele frequencies (EAFs), and p-values, can be found in Supplementary Table SI. Consequently, five methods – IVW, MR-Egger, weighted median, simple mode, and weighted mode – were utilized to analyze the causal relationship between nine blood pressure phenotypes and atrial fibrillation. As shown in Figure 1, our MR analyses revealed potential causal associations between blood pressure phenotypes and AF. In sex-pooled analyses, both DBP (OR = 1.006, 95% CI: 1.002–1.009, p = 0.000640) and SBP (OR = 1.005, 95% CI: 1.002–1.008, p = 0.002285) showed significant associations with AF risk. In sex-specific analyses, significant associations were exclusively identified in females, with both DBP (OR = 1.004, 95% CI: 1.000–1.007, p = 0.049871) and PP (OR = 1.004, 95% CI: 1.001–1.007, p = 0.010487) showing potential causal relationships with AF, while no significant associations were observed in males. After FDR correction, both DBP (OR = 1.006, 95% CI: 1.002–1.009, p = 0.000640) and SBP (OR = 1.005, 95% CI: 1.002–1.008, p = 0.002285) still showed significant associations with AF risk in sex-pooled analyses, as shown in Figures 2 and 3. The analysis results for all phenotypes are detailed in Supplementary Table SII. Sensitivity analyses, including heterogeneity tests and horizontal pleiotropy tests, did not reveal any abnormalities, thereby enhancing the credibility of our results, as indicated in Supplementary Tables SIII and IV. The leave-one-out analysis, depicted in Figure 4, demonstrated that excluding any single SNP did not alter the results, underscoring the robustness of these findings.
Figure 1
Forest plot for the Mendelian randomization (MR) significant association between blood pressure and atrial fibrillation
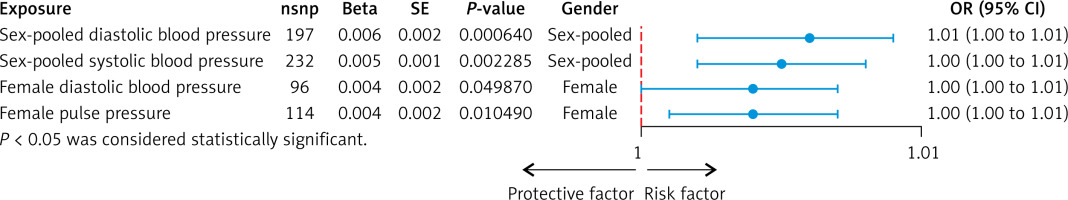
Figure 2
Scatter plot for the Mendelian randomization (MR) association between blood pressure and atrial fibrillation
SNP – single nucleotide polymorphism.
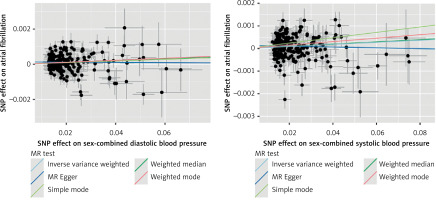
Figure 3
Forest plot for the Mendelian randomization (MR) association between blood pressure and atrial fibrillation. SNP, single nucleotide polymorphism
Figure 4
Leave-one-out plot for the Mendelian randomization (MR) association between blood pressure and atrial fibrillation
SNP – single nucleotide polymorphism.
Our sex-stratified Mendelian randomization analysis provides novel insights into the causal relationship between blood pressure and atrial fibrillation, with notable sex-specific differences. The significant associations found in sex-pooled analyses, particularly for DBP and SBP, align with previous observational studies but provide stronger evidence for causality due to the methodological advantages of MR analysis. Importantly, our sex-stratified analyses revealed a distinct pattern where significant associations were exclusively observed in females, suggesting potential sex-specific mechanisms in the blood pressure-AF relationship.
The female-specific associations between blood pressure parameters (particularly DBP and PP) and AF risk highlight the potential importance of sex-specific pathophysiological pathways. These findings may partially explain the previously observed sex differences in AF outcomes and suggest that females might be more susceptible to blood pressure-induced atrial remodeling. The absence of significant associations in males warrants further investigation and might indicate that other risk factors play more dominant roles in male AF pathogenesis. The robustness of our findings is supported by comprehensive sensitivity analyses, including heterogeneity tests, pleiotropy assessments, and leave-one-out analyses, which collectively strengthen the validity of our conclusions.
In conclusion, our study provides genetic evidence supporting a causal relationship between blood pressure and AF risk, while revealing important sex-specific differences in these associations. These findings have significant clinical implications, suggesting that blood pressure management might be particularly crucial for AF prevention in females. Our results underscore the importance of sex-specific approaches in cardiovascular disease prevention strategies and highlight the need for personalized risk assessment and management protocols. Future research should focus on elucidating the underlying mechanisms of these sex-specific differences to optimize prevention strategies for both males and females.