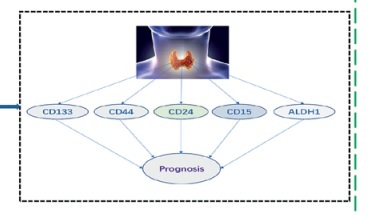
Thyroid cancer is the most common malignant tumor of the thyroid gland [1, 2]. Surgical excision is the predominant therapeutic modality; nevertheless, a subset of advanced patients exhibits an increased propensity for relapse [3]. Thyroid cancer currently lacks practical biomarkers for accurate diagnosis and treatment selection. Puncture biopsy, as a traditional invasive test, exhibits limited patient acceptance and poses a risk of spreading cancerous tissue. In recent years, numerous studies have demonstrated a close association between cancer stem cells (CSCs) and tumor initiation and progression [4–6]. Therefore, it is of great significance to identify the critical markers of thyroid cancer stem cells (TCSCs) for prognostic prediction. In this study, we systematically reviewed the literature to determine the significance of TCSC markers for thyroid cancer prognosis (Figure 1).
Figure 1
Research flow chart to investigate the relationship between cancer stem cell markers and thyroid cancer prognosis
Methods
Search strategy
The literature search on CSC markers and the prognosis of thyroid cancer was conducted using Embase, PubMed and Web of Science databases. The search deadline was December 2023.
Inclusion criteria
(1) Subjects with thyroid cancer diagnosed pathologically or histologically; (2) The relationship between TCSC markers and the prognosis of thyroid cancer was reported; (3) Survival-related data were reported directly or indirectly in the literature; (4) The study was retrospective and the language was limited to English.
Exclusion criteria
In vitro or animal experiments; Studies with limited or inadequate data; Duplicate published studies; Non-original studies, such as systematic reviews, meta-analyses, and conference abstracts.
Data extraction and study selection
Two authors independently extracted data from the included literature and cross-checked the data. The information extracted mainly included: the first author, year of publication, country, thyroid tumor type, sample size, CSC markers, detection methods, cut-off values, outcome indicators, follow-up time and literature quality scores.
Results
Literature search results
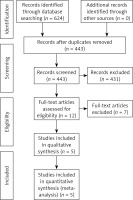
A total of 624 articles were obtained through the preliminary search. A total of 619 articles were excluded through the literature retrieval process shown in Figure 2, and 5 articles [7–11] with 1478 patients were finally included. The basic characteristics of the included articles are shown in Table I. The TCSC markers involved were CD133, CD44, CD24, CD10, CD15 and ALDH1.
Table I
Basic characteristics of included studies
Relationship between cancer stem cell markers and prognosis of thyroid cancer
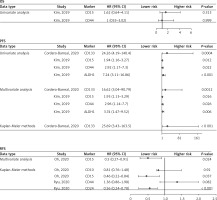
Oh et al. [8] demonstrated that elevated CD15 expression was correlated with improved recurrence-free survival (RFS) (Kaplan-Meier methods: hazard ratio (HR) = 0.46, 95% confidence interval (CI) (0.22–0.84), p = 0.037; Multivariate analysis: HR = 0.50, 95% CI (0.27–0.91), p = 0.024). However, elevated CD10 expression was not correlated with improved RFS (Kaplan-Meier methods: HR = 0.81, 95% CI (0.76–1.48), p = 0.920) (Figure 3).
Figure 3
Forest plot of the relationship between cancer stem cell markers and prognosis of thyroid cancer
Bi et al. [9] demonstrated that elevated CD44 expression was correlated with poor overall survival (OS) (Univariate analysis: p = 0.030) and disease-free survival (DFS) (Univariate analysis: p = 0.017). The elevated CD133 expression was correlated with poor OS (Univariate analysis: p = 0.046) but not DFS (p = 0.799).
Ryu et al. [11] demonstrated that elevated CD24 expression was correlated with improved RFS (Kaplan-Meier methods: HR = 0.56, 95% CI (0.24–0.78), p < 0.001) (Figure 3). The CD44 expression was not correlated with RFS (Kaplan-Meier methods: HR = 1.36, 95%CI (0.86–1.98), p = 0.082) (Figure 3).
Kim et al. [10] showed that the expressions of CD15 and CD44 were not associated with OS (Univariate analysis: HR = 1.62, 95% CI (0.6–4.11), p = 0.313; HR = 1.00, 95% CI (0.33–3.02), p = 0.999) (Figure 3). The elevated expressions of CD15, CD44 and ALDH1 were correlated with poor PFS (Univariate analysis: HR = 1.94, 95% CI (1.16–3.27), p = 0.012; HR = 2.92, 95% CI (1.17–7.30), p = 0.022; HR = 7.24, 95% CI (3.11–16.86), p < 0.001; Multivariate analysis: HR = 1.93, 95% CI (1.13–3.29), p = 0.016; HR = 2.96, 95% CI (1.14–7.70), p = 0.026; HR = 3.74, 95% CI (1.47–9.52), p = 0.006) (Figure 3).
Cordero-Barreal et al. [12] demonstrated that elevated CD133 expression was correlated with poor PFS (Univariate analysis: HR = 24.259, 95% CI (4.19–140.4), p = 0.0004; Multivariate analysis: HR = 16.619, 95% CI (3.04–90.79), p = 0.0012; Kaplan-Meier methods: HR = 25.69, 95% CI (3.43–163.5), p < 0.001) (Figure 3).
Discussion
CD24, CD15, CD44, CD133 and ALDH1 were considered candidate CSCs markers. Bi et al. [9] conducted a 10-year follow-up analysis of patients with CD133 positivity in MTC, revealing that high expression of CD133 is associated with poor prognosis and can serve as an independent prognostic indicator for medullary thyroid carcinoma (MTC) patients. Research has shown a significant correlation between CD133 and adverse prognosis in MTC [12]. Friedman observed a significantly higher tumorigenicity of CD133+/high cells in nude mice compared to CD133+/low cells, and even CD133-cells failed to initiate tumor formation [13]. CD133 serves not only as a reliable marker of TCSCs, but also exerts a significant regulatory influence on the progression of thyroid cancer. Wang et al. demonstrated that CD133 enhances the expression of SLC1A3, thereby promoting the self-renewal ability of TCSCs [14]. The overall prognosis of MTC patients is worse than that of other differentiated thyroid patients, primarily attributed to its inherent resistance towards radioactive iodine treatment [15]. Compared with CD133-negative cells, CD133-positive cells are more effective in treating radioactive iodine [16]. It promotes future recurrence and metastasis and ultimately leads to an unfavorable prognosis. CD44, a recognized marker of CSC, is a crucial regulator of epithelial-mesenchymal transformation and is involved in tumor occurrence, progression, and metastasis. In recent years, there has been a continuous influx of studies focusing on CD44 in TCSCs. Our systematic review found that CD44 was an essential predictor of OS and DFS at MTC. CD133 combined with CD44 helps predict prognosis in colorectal patients. Bi et al. [9] also found that CD133 and CD44 may be prognostic factors for OS and DFS in patients with MTC. CD44+/CD24- cells are present in thyroid papillary carcinoma, and CD44+/CD24- papillary carcinoma cells highly express OCT4 and the transcription factor POU5F1. Ahn et al. [17] identified CD44+/CD24- cells in human papillary thyroid cancer cells, and the abundance of CD44+ cells exhibited a positive correlation with tumor aggressiveness. Guo et al. [18] demonstrated that human papillary thyroid cancer cells expressing CD44+/CD24- exhibited enhanced proliferative capacity and resistance to 5-FU compared to CD44-/CD24- cells [18]. Ryu et al. [11] showed that CD44+/CD24- was identified as an independent prognostic factor for PTC with a hazard ratio of 4.207. ALDH1 plays a vital role in mediating chemotherapeutic drug resistance and cell detoxification. However, the prognostic and clinical significance of ALDH1 in thyroid cancer remains unclear. This systematic review found that high expression of ALDH1 is associated with PFS and poor PTC. Furthermore, Xing et al. [19] demonstrated that the expression of ALDH1 served as an independent prognostic factor for both lymph node recurrence-free survival and distant recurrence-free survival in patients with PTC. In addition, Cui et al. [20] conducted a comprehensive analysis of the public database and demonstrated that the transcriptional expression of ALDH1 serves as an independent prognostic factor for thyroid cancer, thereby suggesting its potential utility as a prognostic biomarker in the context of thyroid cancer treatment. Additionally, ALDH serves as a pivotal marker for identifying CSCs and plays a crucial role in governing various cellular processes encompassing self-renewal, expansion, differentiation, as well as resistance to therapeutic agents and radiation. CD10 is highly expressed in anaplastic thyroid carcinoma but lowly expressed in other types of thyroid carcinoma. CD15 expression is seen in various types of thyroid cancer but not in normal thyroid tissue or benign thyroid tumors. This conclusion is supported by Oh et al. [8]. In addition, high expression of CD15 was associated with poor RFS of PTC; this conclusion is supported by Kim et al. [10]. Overall, the above TCSCs markers are associated with the prognosis of thyroid cancer. Consequently, the detection of these markers holds potential for facilitating personalized and precise treatment strategies in patients diagnosed with thyroid cancer.
In conclusion, to the authors’ knowledge, this is the first systematic review of TCSC markers as a prognostic factor for thyroid cancer. According to the current study, five TCSC markers (CD133, CD44, CD24, CD15 and ALDH1) are potential candidates for predicting the prognosis of thyroid cancer. The limited number of studies, different TCSC markers, types of prognostic indicators, and sources of survival data preclude the possibility of conducting a quantitative meta-analysis. Therefore, further investigation is warranted to conduct high-quality studies with improved homogeneity in order to quantitatively elucidate the association between TCSC markers and the prognosis of thyroid cancer.