Introduction
Central vein access devices (CVADs) are catheters equipped with catheter tips positioned in the central vein [1]. Currently, the commonly employed CVADs in clinical practice encompass central venous catheters (CVCs), peripherally inserted central catheters (PICCs) and totally implantable venous access ports (TIVAPs) [2]. CVADs are extensively utilized in critically ill patients and cancer patients necessitating multiple chemotherapy regimens [3]. The clinical application of CVADs has led to an increasing prominence of complications, including catheter thrombosis, puncture site bleeding, catheter slippage, and catheter-related bloodstream infections (CRBSIs) [4]. The occurrence of CRBSIs represents a significant and consequential complication [5]. CRBSIs are defined as the occurrence of bacteremia or fungemia within 48 h of intravascular catheter insertion or withdrawal, accompanied by infection manifestations such as fever (greater than 38°C), chill or hypotension, and absence of any other identifiable source of infection apart from vascular catheter-associated infection [6, 7]. The occurrence of CRBSIs not only impacts patient prognosis but also significantly elevates mortality rates and hospitalization costs [8]. Treatment expenses for CRBSIs range from $32,000 to $69,332 [9–11]. Furthermore, patients with CRBSIs face a 2.71-fold higher risk of mortality compared to those without this condition [12].
The incidence of CRBSIs varies among different types of CVADs. In patients with PICCs, the reported incidence ranged from 0.46% to 13.4% [13–15], while in patients with CVCs, the reported incidence ranged from 1.88% to 23.53% [16–18], and the incidence of CRBSIs in TIVAPs patients ranged from 1.32% to 13.02% [13, 17, 19]. The incidence of CRBSIs was found to be higher in patients with TIVAPs compared to those with PICCs, as demonstrated by a meta-analysis [20]. A meta-analysis conducted by Chopra et al. revealed that PICCs had a lower risk of CRBSIs compared to CVCs [21]. Another meta-analysis conducted by Capozzi et al. [22] revealed no statistically significant difference in the incidence of CRBSIs between TIVAP and PICC patients. The literature included in these meta-analyses primarily consisted of retrospective studies, which are associated with numerous confounding factors. Consequently, there may be limitations regarding the reliability and accuracy of the data derived from these studies. Existing randomized controlled trials (RCTs) [23–26] or meta-analyses [20–22] have solely compared the incidence of CRBSIs between two types of these CVADs, failing to provide a comprehensive and clear comparison among various CVADs, thereby hindering optimal clinical decision-making.
While traditional paired meta-analyses are limited to comparing only two interventions, network meta-analysis enables simultaneous comparison of multiple interventions and provides a quantitative ranking of different outcome measures based on the likelihood of advantages and disadvantages [27, 28]. In this study, a systematic review and network meta-analysis of RCTs were conducted on the incidence of CRBSIs in various types of CVADs, with the objective of providing an evidence-based foundation for selecting the most optimal CVADs (Supplementary Figure S1).
Material and methods
This network meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement extension for network meta-analysis [29].
Inclusion criteria
Search strategy
The incidence of CRBSIs in different types of CVADs was investigated through a comprehensive search of RCTs from PubMed, EMBASE, Web of Science, Cochrane, CNKI and CBM databases. MeSH terms were combined with free words to optimize the search strategy. Additionally, manual tracing of references in the included literature was conducted. The search period extended until May 31, 2024. The search terms included catheter-related bloodstream infection, central venous access device, central venous catheter, peripherally inserted central catheter, totally implantable venous access port, random*, etc.
Literature screening and data extraction
The literature was imported into the Endnote software for deduplication purposes. Two researchers independently screened the literature, and any disagreements were resolved through discussion with a third researcher. Initially, the titles of the literature were read to exclude obviously irrelevant studies. Subsequently, both abstracts and full texts were reviewed for further filtering. Relevant data including first author, publication date, study design type, study start time, sample size, catheter type, and number of CRBSI cases were extracted from the selected literature.
Risk of bias assessment
The quality of the literature was assessed by two researchers in accordance with the RCT bias risk assessment tool recommended by the Cochrane reviewers’ handbook. The evaluation primarily encompassed seven aspects: random sequence generation, allocation concealment, blinding of both researchers and subjects, blind evaluation of outcomes, integrity of outcome data, selective reporting and other potential biases.
Statistical analysis
Direct pairwise meta-analyses were performed using RevMan 5.3 software, and heterogeneity was tested. Risk ratio (RR) was employed as the effect size for the count data, with each effect size presented along with the 95% confidence interval (CI). Stata 14.0 software was utilized to perform network meta-analysis based on the frequency framework. Network evidence plots were drawn for comparison between each outcome measure intervention. In the case of a closed loop in the network evidence plots, node analysis was applied to test the inconsistency. If p > 0.05, a consistency model was used for analysis. The surface under the cumulative ranking curve (SUCRA) was used to rank outcome indicators accordingly. A “comparison-adjusted” funnel plot was employed to assess potential publication bias.
Results
Study selection, characteristics and risk of bias assessment
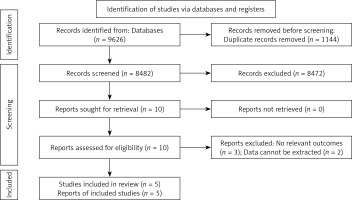
A preliminary search yielded 9626 relevant literatures sources, and after a gradual screening process (Figure 1), five studies [23–26, 30] were ultimately included. The included studies were published between 2014 and 2021 and all of them were RCTs. Two studies were conducted in China, while the remaining three originated from Sweden, Italy and the UK. The number of catheters involved ranged from 23 to 303. Table I presents the essential characteristics of the included literature sources. Four studies employed appropriate randomization methods, while one study had an unclear randomization approach. Three studies concealed their allocation scheme effectively, while it remained unclear in two other studies. Regarding blinding method, although the results were not explicitly stated, due to challenges in achieving double or triple blinding for CVAD placement evaluation purposes, this aspect was excluded from the scope of literature quality reference to enhance risk control within the articles. Other aspects exhibited low risk bias levels. The risk of bias in included studies is shown in Figure 2.
Table I
Characteristics of interventions of included studies
Pairwise meta-analyses
PICCs versus CVCs
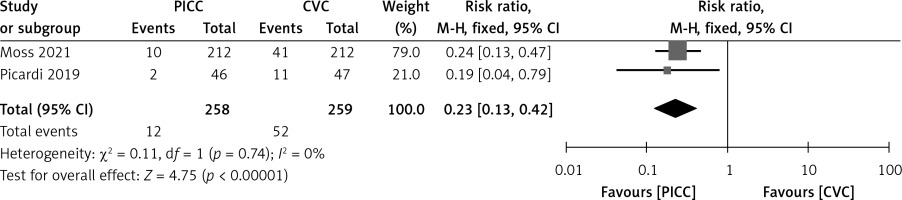
Two studies compared the incidence of CRBSIs between the PICC group and the CVC group. The heterogeneity test result was: I2 = 0%, p = 0.74; therefore a fixed-effect model was adopted. The meta-analysis demonstrated a statistically significantly lower incidence of CRBSIs in the PICC group compared to the CVC group (RR = 0.23, 95% CI (0.13–0.43), p < 0.00001) (Figure 3).
PICCs versus TIVAPs
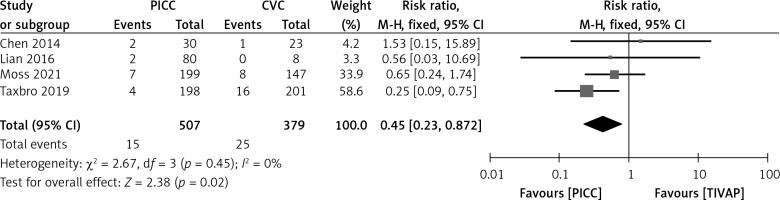
Four studies compared the incidence of CRBSIs between the PICC group and the TIVAP group. The heterogeneity test result was: I2 = 0%, p = 0.45; therefore a fixed-effect model was adopted. The meta-analysis demonstrated a statistically significantly lower incidence of CRBSIs in the PICC group compared to the TIVAP group (RR = 0.45, 95% CI (0.23–0.87), p = 0.02) (Figure 4).
Network meta-analysis
Evidence network diagram
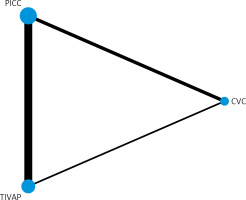
The occurrence of CRBSIs was reported in five RCTs involving three types of CVADs. In the figure, each dot represents a specific CVAD, while the thickness of the line connecting two points indicates the corresponding sample size. A thicker solid line signifies a greater amount of direct comparative evidence, whereas a thinner line suggests less evidence in that regard. Notably, it can be observed that PICCs exhibit both the largest number of relevant studies and sample size (Figure 5).
Inconsistency test
The inconsistency test was performed using node analysis, and the result indicated the absence of any significant inconsistencies (p > 0.05). This indicated that the findings from direct comparison align with those obtained through indirect comparison.
Network meta-analysis results of CRBSIs
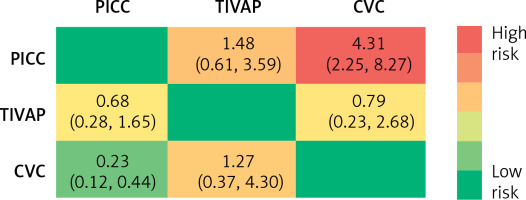
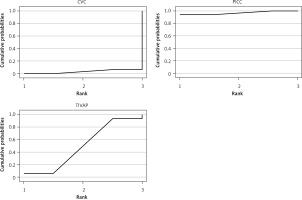
The incidence of CRBSIs was significantly lower in the PICC group compared to the CVC group. No significant differences were observed in other comparisons (Figure 6). Based on SUCRA values, the ranking of three CVADs was as follows: PICCs (97.20%) > TIVAPs (50.00%) > CVCs (2.80%) (Figure 7).
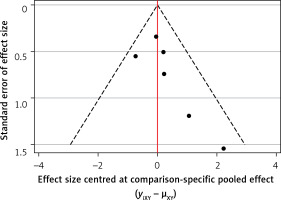
Publication bias analysis
The findings revealed a non-uniform distribution of all study sites across both sides of the median line, indicating a lack of symmetry and suggesting potential publication bias (Figure 8).
Discussion
As an invasive procedure, CVADs are susceptible to complications [31]. CRBSIs represent a significant complication [32]. The presence of a venous indwelling catheter compromises the integrity of the skin, allowing pathogens to invade and proliferate along the catheter, leading to bloodstream infection or even systemic infection. This poses a serious threat to patient health, resulting in prolonged hospital stays, increased mortality rates, and escalated healthcare costs [31, 33–35]. Therefore, CRBSIs serve as a crucial indicator for nosocomial infection prevention and control and have garnered considerable attention in clinical practice. The incidence of CRBSIs varies depending on different infusion tools; thus, selecting appropriate CVADs is paramount when considering CRBSI occurrence.
The present study conducted a systematic analysis comparing the incidence of CRBSIs among PICCs, CVCs, and TIVAPs. Both direct pairwise meta-analyses and network meta-analysis results consistently demonstrated that the PICC group had a lower incidence of CRBSIs compared to the CVC group. From the perspective of SUCRA probability ranking, the PICC group ranks first. Previous meta-analyses have found a reduced risk of CRBSIs in PICCs compared to CVCs [21]. Another meta-analysis showed the same results [36]. This difference in incidence of CRBSIs between the PICC group and the CVC group may be attributed to variations in puncture locations; predominantly the upper limbs for PICCs versus the neck and subclavicular region for CVCs. The skin on the upper limb is less prone to bacterial colonization, sweat accumulation, and oily secretions than that on the neck and subclavicular region, thereby contributing to higher incidence of CRBSIs observed in the CVC group [14].
The TIVAP group also exhibited a significantly lower incidence of CRBSIs than the CVC group, with a more pronounced disparity observed when compared to the PICC group. TIVAPs offer durable venous access and employ a closed intravenous infusion system, thereby mitigating complications, particularly those related to infection [37]. TIVAPs represent an entirely implanted closed intravenous infusion device that remains subcutaneously placed within the human body [38]. This technology boasts advantages such as minimal risk of infection, enhanced quality of life convenience, simplified maintenance requirements, and prolonged service life [39]. Since a TIVAP is an intravenous infusion device that is completely implanted under the skin and has no exposed part, the entire device has less direct contact with the external environment, reducing the incidence of CRBSIs [40, 41]. However, the PICC catheter is exposed at the elbow, which requires regular dressing change and tube flushing, and skin colonizing bacteria can easily migrate into the blood vessels, resulting in the occurrence of CRBSIs [20, 23]. When using PICCs for infusion, blood drawing, tube flushing and other operations, there is a potential risk of introducing microorganisms into the catheter lumen. Notably, the manipulation of the catheter hub represents the most prevalent source of infection [20]. In direct comparison based on meta-analytical findings, the incidence of CRBSIs was observed to be lower in the PICC group compared to the TIVAP group, which is consistent with the findings of another meta-analysis [20]. The results of the network meta-analysis comparison showed no difference between the two. From the perspective of SUCRA probability ranking, the PICC group ranks ahead of the TIVAP group. In RCTs involving a large sample of solid tumors, the incidence of CRBSIs in TIVAPs was lower than in PICCs [30]. Conversely, in RCTs with a large sample size of blood tumors, the incidence of CRBSIs was higher in TIVAPs compared to PICCs [26]. Additionally, in RCTs focusing on long-term parenteral nutrition, the incidence of CRBSIs was higher in TIVAPs compared to PICCs [23, 24]. Infusion of parenteral nutrition with TIVAPs increases the risk of catheter-associated infections. This may be due to the fact that parenteral nutrition itself, both lipids and amino acids, are conducive to bacterial colonization and biofilm formation, or that the procedure required for parenteral nutrition infusion is more frequent [42–44]. For patients with solid tumors, TIVAPs may represent a preferable option.
Although the incidence of CRBSIs varies among different types of CVADs, it is crucial to consider other risk factors that contribute to the high risk of CRBSIs. Previous studies have demonstrated that diabetes, the use of antibiotics, a long-term indwelling urinary catheter (> 7 days), the use of antibiotics, advanced age (> 55 years), and a higher Acute Physiology and Chronic Health Evaluation (APACHE) score are high-risk factors for the development of CRBSIs [45]. Therefore, we recommend constructing relevant risk prediction models to identify high-risk groups for CRBSIs and implementing targeted interventions promptly, which will effectively reduce the incidence of CRBSI. In order to better control the occurrence of CRBSIs, we recommend the use of some effective measures, such as the use of antibacterial coating restraint tubes, and strict cleaning, disinfection and puncture procedures. When intravenous therapy teams or nurses perform standardized and standardized nursing work, the infection rate will be greatly reduced, from 25–33% to 4% on average, or even lower [46, 47]. The guidelines state that all healthcare workers inserting catheters should receive formal insertion training and strictly adhere to aseptic procedures [48].
Advantages. The present network meta-analysis represents the first attempt to compare the incidence of CRBSIs among three types of CVADs, yielding a relatively robust conclusion. First of all, in terms of incidence of CRBSIs, PICCs outperformed both CVCs and TIVAPs, thus demonstrating their potential clinical value and guiding significance. This study provides a scientific basis for the selection of PICCs, CVCs and TIVAPs catheters for CVADs. Furthermore, these findings offer valuable guidance for clinicians when making decisions regarding treatment options. Secondly, this study included five high-quality RCTs, ensuring its representativeness and credibility. Lastly, by employing network meta-analysis and SUCRA probability ranking techniques, this study enhances objectivity and comprehensiveness in its results while providing more accurate references for clinical practice.
Limitations. First of all, there are variations in the number of included studies across different CVADs. Some literature exhibits a limited number of CVADs and small sample sizes. Therefore, to ensure the reliability and objectivity of the conclusions, it is imperative to confirm their scientific nature through multi-center RCTs with large samples and high-quality collaboration. Secondly, although all included subjects were adult patients with CVAD placement, differences in regional medical expertise and hospital capabilities as well as variations in intervention programs’ intensity may contribute to result heterogeneity. Third, the included studies were published in both Chinese and English literature; however, some publications might be incomplete. Fourth, CRBSIs are related to the catheter type, and other factors such as total parenteral nutrition, chemotherapy, and use of immunosuppression are also related, which may lead to certain bias in this meta-analysis.
In conclusion, the limited evidence suggests that the incidence of CRBSIs with PICCs is lowest, followed by TIVAPs. Therefore, when selecting CVADs, PICCs should be prioritized based on these findings, which offer valuable clinical guidance. However, it is important to interpret these results cautiously due to the limitations in the number and quality of included studies and literature. Further high-quality direct comparative randomized controlled trials are needed to provide more reliable references for clinical applications.