Methotrexate (MTX) administered at the dose of 10–15 mg/m2 is currently recommended as the first-line disease-modifying agent in most juvenile idiopathic arthritis (JIA) subtypes [1].Despite the prediction of early MTX treatment response and development of drug adverse effects is crucial to individualize JIA therapy and in consequence prevent long-term effects of inadequate treatment, up to date we are not able to predict whether the patient benefits from MTX therapy [2]. Single nucleotide polymorphisms (SNPs) in the genes involved in the MTX cellular pathway are candidate biomarkers of MTX toxicity and treatment response, but currently available studies failed to establish a definite link [3, 4].
Folate metabolism is perceived as the major target of MTX anti-inflammatory action. As a competitive inhibitor of dihydrofolate reductase, MTX prevents the regeneration of tetrahydrofolate, which is essential for the generation of folate cofactors required for de novo purine and pyrimidine synthesis. Moreover, MTX may influence several other steps in folate metabolism, leading to cellular folate depletion and inhibition of methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTR), and methionine synthase reductase (MTRR), which play pivotal roles in both folate and homocysteine metabolism [5]. Genetic variability within folic acid metabolism genes has been associated with MTX treatment efficacy and the presence of therapy side effects both in rheumatoid arthritis (RA) and JIA [6, 7], although, due to small sample sizes and differences in study design, currently available data are inconclusive.
The aim of our study was to identify the association between genetic variability within MTHFR rs1801133 (C677T) and MTRR rs1801394 (A66G) genes and the disease activity, MTX treatment efficacy, and the presence of therapy side effects in Polish JIA patients.
Methods
Patients and study design
The inclusion criteria were the fulfilment of International League Against Rheumatism (ILAR) JIA diagnostic criteria [8] and current or past MTX treatment. MTX was administered once a week at the recommended dose (10–15 mg/m2 of body surface area – BSA) by either oral or subcutaneous route. All patients were receiving folic acid supplementation. Demographic, clinical (number of joints with active arthritis and limited range of motion, Childhood Health Assessment Questionnaire (CHAQ) value, Juvenile Arthritis Disease Activity Scale (JADAS) [9] value), and laboratory parameters (C-reactive protein [CRP], erythrocyte sedimentation rate (ESR)) were collected at the moment of JIA diagnosis and on a control visit 4–6 months (median: 5.09 months) after MTX treatment initiation (Table I). Therapy adverse effects (AEs) were evaluated at the control visit. AEs were classified as gastrointestinal (nausea, vomiting, abdominal pain), hepatotoxicity (defined as alanine aminotransferase and/or aspartate aminotransferase activity exceeding normal range upper limit), dermatological, neurotoxicity, recurrent infections, and other AEs.
Table I
The influence of MTHFRrs1801133 and MTRR rs1801394 polymorphism on JIA activity
[i] MTX – methotrexate, BSA – body surface area, PGA – Physician’s Global Assessment of Disease Activity, PGE – parent’s general evaluation of well-being, CHAQ – Childhood Health Assessment Questionnaire, ROM – range of motion, ESR – erythrocyte sedimentation rate, CRP – C-reactive protein, JADAS-71 – Juvenile Arthritis Disease Activity Score including 71 joints.
Genotyping
Genomic DNA was isolated with the QIAamp DNA Mini Kit and assessed by ultraviolet spectrophotometry. Genotyping was performed with TaqMan GTXpress Master Mix and dedicated TaqMan SNP Genotyping Assay (MTHFR rs1801133, MTRR rs1801394). For allelic discrimination, SDS Software 2.0 was used.
Statistical analysis
Continuous variables are presented as the median with the interquartile range and assessed using the Mann-Whitney U test. Categorical variables are presented as the number with an appropriate percentage, and compared using the χ2, χ2 with Yates correction, and Fisher exact tests on an individual basis. The logistic regression model was used to identify associations between SNPs and the occurrence of the side effects when adjusted to the age at disease diagnosis, sex, dose, and route of MTX administration. A p-value < 0.05 was considered statistically significant. Both MTHFR rs1801133 and MTRR rs1801394 were in Hardy-Weinberg equilibrium.
Results
The study group consisted of 100 JIA patients: 77 girls and 23 boys with a median age of 8.26 (4.15–11.10) years. 64% of patients were diagnosed with oligoarthritis, 17% with polyarticular seronegative JIA subtype, and 2% with polyarticular seropositive JIA subtype. Enthesitis-related arthritis was present in 8% of the study population, followed by systemic arthritis (6%) and psoriatic arthritis (3%). The median dose of MTX was 10.71 (9.80–12.07) mg/m2 BSA.
At the moment of JIA diagnosis patients with MTHFR rs1801133 CT/TT variant had significantly higher values of inflammatory markers (ESR 34.00 mm/h vs. 16.00 mm/h, p = 0.02; CRP 8.00 mg/l vs. 3.20 mg/l, p = 0.02), a higher number of joints with active arthritis (2.00 vs. 1.00, p = 0.04), and higher Juvenile Arthritis Disease Activity Score (JADAS) 71 value (14.50 vs. 11.40, p = 0.02) in comparison with CC individuals. Children with MTRR rs1801394 AG/AA genotype presented significantly higher inflammatory markers values at the baseline of MTX therapy than GG individuals (ESR 26.00 mm/h vs. 8.00 mm/h, p < 0.01; CRP 8.00 mg/l vs. 0.70 mg/l, p < 0.01) (Table I). We did not observe any association between the studied SNPs and the efficacy of MTX treatment assessed at the control visit. There was no statistically significant association between the occurrence rate of MTX therapy side effects and the frequency of the investigated SNPs, although, after adjustment for sex, dose, and route of MTX administration, patients with MTHFR rs1801133 CT/TT variant had 3.3-fold greater likelihood of hepatotoxicity development, with the p-value at the border of statistical significance (OR = 3.3, 95% CI: 0.95–11.53, p = 0.06).
Discussion
Our study demonstrates an association between SNPs rs1801133 in MTHFR and rs1801394 in MTRR genes and the JIA inflammatory activity at the moment of disease diagnosis. To the best of our knowledge, this is the first study to reveal this relationship. We hypothesise that this association is the effect of increased plasma concentration of homocysteine in individuals with studied SNPs.
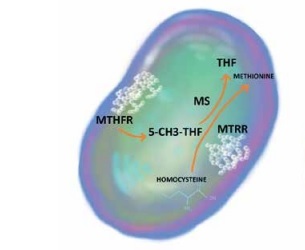
As the key enzyme in the folic acid cycle, MTHFR is essential for the conversion of homocysteine to methionine. SNP rs1801133, a missense variation from cytosine to thymine, attenuates its catalytic activity, which leads to hyperhomocysteinaemia. Furthermore, an increase in the plasma level of homocysteine is also the result of MTRR polymorphism. SNP rs1801394 alters isoleucine into methionine in the protein chain and subsequently disrupts the methionine/homocysteine cycle [10, 11]. The impact of the homocysteine concentration on the degree of inflammation in the course of autoimmune diseases has been demonstrated in several studies (Figure 1).
Figure 1
The impact of MTHFR rs1801133 and MTRR rs1801394 on the methionine/homocysteine cycle
5-CH3-THF – 5-methyltetrahydrofolate, MS – methioninesynthase, MTHFR – methylenetetrahydrofolatereductase, MTRR – 5-methyltetrahydrofolate-homocysteine methyltransferase reductase, THF – tetrahydrofolate.
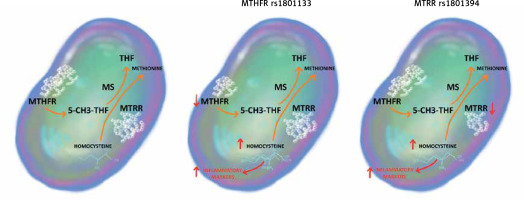
The most convincing data come from the research conducted on patients with RA, where the positive relationship between the concentration of homocysteine and proinflammatory markers (human soluble interleukin 2 receptor – IL-2sRα, soluble tumour necrosis factor α receptor – sTNF-R75), adhesion molecules (intercellular adhesion molecule 1 – sICAM-1), and CRP, along with clinical features of RA (disease activity, number of joints with active inflammation), was observed [12]. Moreover, the concentration of homocysteine in the synovial fluid of RA patients is significantly higher than in healthy individuals, leading to enhanced interleukin-6 and interleukin-8 production in synoviocytes and nuclear factor κB (NF-κB) activation in rheumatoid cells [13]. Meanwhile, studies conducted in children with JIA revealed comparable results, indicating an increase in the serum concentration of homocysteine in comparison to healthy controls [13] and in patients with a polyarticular subtype of JIA in comparison to children with oligoarthritis [14]. No correlation between the homocysteine concentration and administration of MTX was established [15]. Genotyping of MTHFR rs1801133 and MTRR rs1801394 may become a useful tool in everyday practice of the paediatric rheumatologist, leading to early recognition of patients at risk of greater disease activity and therefore personalisation of JIA treatment in order to achieve early disease remission, as advised in the treat-to-target strategy.
Because JIA is a rare disease, the sample size was relatively small, and the lack of positive associations between clinical response to MTX and investigated genetic variants may be the effect of low study power. Furthermore, because the primary aim of our research was the evaluation of SNPs within the MTX cellular pathway genes’ influence on MTX early efficacy, the measurement of plasma homocysteine concentration in our study group was not performed.
In conclusion, JIA patients with MTHFR rs1801133 and MTRR rs1801394 genotypes present higher disease activity than children with wild-type alleles, and hence they require a more intensive treatment and follow-up strategy at the moment of JIA diagnosis. Due to the relatively small study group and lack of homocysteine concentration measurement, our results should be interpreted as preliminary.