Introduction
Cryptorchidism (undescended testis – UDT) is the most common congenital anomaly of the urogenital system in males, affecting 2–8% of full-term boys [1, 2]. UDT may be situated in any position along its normal route of descent and can be palpable or non-palpable when examined manually. Non-palpable UDT means that the testis is either intra-abdominal (IAT) or absent (agenesis or vanishing testis) [1, 2].
Out of two modes of treatment (hormonal and surgical) surgical intervention (orchidopexy) is recommended as the primary approach in boys with UDT [3–7]. The European Society for Paediatric Urology (ESPU)/European Association of Urology (EAU) [7], the American Urological Association (AUA) [4], the Canadian Urological Association (CUA) [6] and the British Association of Paediatric Surgeons (BAPS)/British Association of Paediatric Urology Surgeons (BAPU)/Royal College of Surgeons (RCS) [5] do not recommend routine hormonal treatment for non-palpable UDT. Adjuvant endocrine treatment with GnRH analogues is recommended for boys with bilateral UDT to preserve the fertility potential [7].
Laparoscopy has become widely used to diagnose impalpable testes since 1976 [8]. Using laparoscopy, the testes can be clearly identified and localized, and treatment can be administered immediately after making a diagnosis. Laparoscopy is recommended because of its excellent sensitivity and specificity in identifying an intra-abdominal testis [4–7, 9].
The IAT creates a technical challenge for a surgeon due to the distance between the internal inguinal ring and scrotum. The approach to the IAT has changed over the years. The Fowler and Stephens maneuver with testicular vessel ligation for orchidopexy was a milestone in treatment of high UDT [10]. Thirty years later, Bloom adopted their idea and reported an initial experience of laparoscopic ligation of the testicular vessels followed by a staged open orchidopexy [11]. Nowadays the two-stage laparoscopic Fowler-Stephens (F-S) operation (2SLF-SO) has become a routine procedure for IAT and is recommended by the national experts’ consensuses and guidelines [3–5, 7].
The most alarming complication of orchidopexy is testicular atrophy, which occurs when the testicular vessels are damaged. The aim of our study was to evaluate testicular growth and risk of atrophy at different lengths of time from the 2SLF-SO in boys with abdominal cryptorchidism.
Material and methods
Examined group
In the years 2011–2017 an average of 3453 surgical patients were hospitalized annually in the Pediatric Center of the Medical University in Lodz, Poland, of which 61.7/year on average were boys with UDT (range: 1.5–2.7%; mean: 1.8%). There were 27 boys with non-palpable UDT among them, on average 3.9 patients annually (range: 1.8–11.9%; mean 6.3% of all patients with UDT) (Table I). All patients with the non-palpable UDT first referred to the clinic were included in the study. A retrospective analysis was performed of the medical records of these boys.
Table I
Number of surgical patients hospitalized in the years 2011–2017 in the Pediatric Center of the Medical University in Lodz, Poland, and number of patients with undescended testis (UDT)
Approval for the study was obtained from the Bioethics Committee of the Medical University of Lodz (No. RNN/265/18/KE of June 10, 2018).
Methods
All 27 boys were qualified for laparoscopy after initial ultrasound (US) examination of the testes. In 21 boys with 29 IAT the laparoscopic F-S operation was performed [10, 11], followed by inguinal orchidopexy 9–12 months later [12]. Boys with bilateral IAT underwent a simultaneous bilateral laparoscopic F-S procedure, followed by inguinal orchidopexy first of the bigger or lower testis, and of the other testis 3 to 6 months later.
The F-S operation is a laparoscopic intraperitoneal ligature and division of the testicular vessels which leaves the testis dependent on the vessel, cremasteric and gubernacular arteries.
Clinical and ultrasound (US) examination was performed at six time points: before treatment (TP1), 3 months (TP2) and 9–12 months after the laparoscopic F-S operation (TP3), 3 months after inguinal orchidopexy (TP4), 3–6 years of age (TP5) and 7–9 years of age (TP6). The last two time points were chosen on the basis of the developmental changes in the testes [13]. At the first four time points 29 testes of 21 boys of the study group were measured, while at TP5 17 gonads and at TP6 8 gonads were assessed depending on when these boys started treatment.
Three dimensions of both testes were recorded by means of scrotal US (Philips iU22, linear transducer 12 MHz, Netherlands) and used to calculate testicular volume (TV): TV (cm3/ml) = 0.52 × width (cm) × length (cm) × height (cm). The testicular atrophy index (TAI) of the affected testicle was calculated: TAI (%) = [(contralateral TV – affected TV)/contralateral TV] × 100% [2]. Atrophy of the testis was considered when TAI was above 50%.
Statistical analysis
The TV and TAI values at the consecutive time points were subjected to statistical analysis. The Shapiro-Wilk test was used to verify normal distributions of data. Variance homogeneity was verified with the Brown-Forsythe’s test, while Mauchly’s test was employed to test for variance sphericity. Numerous tested variables did not meet the assumptions of parametric tests (normal distribution, variance homogeneity and sphericity); therefore data were presented as the medians and interquartile ranges. The analysis of changes was performed by the nonparametric ANOVA for repeated measurements (Friedman’s test) and the post-hoc multiple comparisons were tested with Dunn’s test. P < 0.05 was considered as significant.
Results
Examined group
Twenty-seven boys aged one to 24 months (mean: 7.1 ±5.1 months, median: 6.0) were treated for non-palpable UDT (35 testes in total), i.e. a testis either in the intra-abdominal position or absent, unilateral or bilateral. All patients were otherwise healthy with no accompanying defects and diseases.
In total, 10 (37%) boys were treated for left, 9 (33.3%) for right and 8 (29.7%) for bilateral non-palpable UDT.
Ultrasound examination before treatment
Out of 35 non-palpable testes 29 gonads were visualized in the intra-abdominal position (25 near internal inguinal ring and 4 a little higher) and 6 testes were not identified during the US examination.
Laparoscopy
During laparoscopy 29 out of 35 testes (82.9%) were found in the intra-abdominal position and subjected simultaneously to the two-stage laparoscopic F-S procedure. 25 testes were classified as low-lying, i.e. < 2 cm above the internal ring, and 4 testes as high-lying, i.e. > 2 cm above the internal ring. In 3 cases (3/35, 8.6%) laparoscopy revealed the rudimentary vessels and vas deferens blind ending in the internal ring (vanishing testis). In the remaining 3 cases (3/35, 8.6%) neither testis nor vessels and vas deferens were found (agenesis of testis).
Analysis of testicular volume
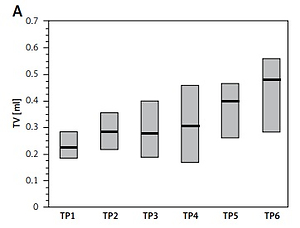
The median volume of the affected testicles before treatment was less than the median TV of the healthy gonad, on average by 26%. Median TV of the affected testes increased systematically at the successive time points, and the increase in testis volume between the second and sixth time points was statistically significant (p < 0.02, R = 2.75) (Table II, Figure 1 A).
Table II
Values of testicular volume (TV) and testicular atrophy index (TAI) at six time points: TP1 – before treatment, TP2 – 3 months and TP3 – 9–12 months after laparoscopy, TP4 – 3 months after orchidopexy, TP5 – 3–6 years and TP6 – 7–9 years of age
| Parameters of the affected testis | Time points (TP) | |||||
|---|---|---|---|---|---|---|
| TP1 N = 29 | TP2 N = 29 | TP3 N = 29 | TP4 N = 29 | TP5 N = 17 | TP6 N = 8 | |
| TV [ml] (median; min.–max.) | 0.22 (0.11–0.70) | 0.28* (0.14–0.70) | 0.28 (0.09–0.63) | 0.31 (0.05–0.65) | 0.40 (0.03–0.96) | 0.48* (0.08–1.17) |
| TAI (%) (median; min.–max.) | 20.9 (–40–73.8) | 12.5 (–25–57.1) | 22.2 (0–80.8) | 42.6 (–16.7–88.9) | 27.3 (–40–62.5) | 10.9 (–57.4–79.1) |
Figure 1
Changes of examined parameters at six consecutive time points (TP1–TP6): A – testicular volume (TV) of the affected testis, B – testicular atrophy index (TAI) of the affected testis
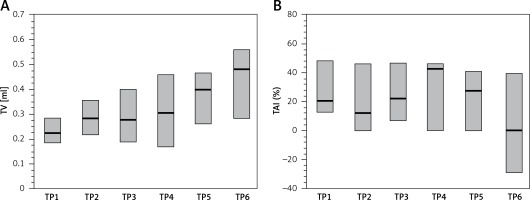
The median TAI value decreased at the subsequent time points from the level of 26% in TP1 to 5.4% in TP6; however, the changes were not statistically significant (Table II, Figure 1 B).
Effectiveness and complications of treatment
After treatment all treated testes were located in the scrotum and no postoperative ascent was observed at any instance.
The overall atrophy rate was 22.9% for the entire group (8/35), but it consisted of two elements: primary atrophy and postoperative atrophy. The first one was connected with a congenital defect and not dependent on the treatment (6/35, 17.1%), while the second one was connected with the treatment (2/29, 6.9%). Therefore, the success rate of treatment was 93.1% (27/29). No other complications were observed.
Both cases of postoperative testicular atrophy were observed at TP4; however, in one instance, subsequent US examination revealed microcalcifications within the undescended testicle. After orchidectomy histopathological examination was performed revealing residuals of the atrophic testis with the predominance of fibrous connective tissue and single degenerated seminiferous tubules.
Discussion
Regardless of the position of IAT in the abdomen, all our patients were treated in two stages with inguinal surgery – orchidopexy – delayed by 6–12 months. In our opinion, this gives time to consolidate the alternative circulation and increases the chance of survival of the treated testicle. In our series of IAT the success rate of treatment by 2SLF-SO was 93%. In the literature success rates of this treatment approach range from 86% to 100% [14–22] and do not differ much from success rates of orchidopexy for canalicular UDT (92.0–97.4%) [23, 24]. After 2SLF-SO testicular viability depends on collateral blood supply from the inguinal canal vessels, gubernacular vessels, and hypertrophy of the artery of the vas. To decrease the incidence of testicular atrophy after the laparoscopic F-S operation, a gubernaculum testis and cremasteric vessels preservation technique was invented recently [15, 16, 20, 22]. However, the results of the laparoscopic F-S operation with or without preservation of gubernacular vessels are comparable (88–100% vs. 88.8–96.7%).
Precise evaluation of the degree of testicular atrophy determines further treatment in a number of patients. In our opinion, reliable assessment can only be done using TV and TAI calculated on the basis of the ultrasound recordings [2, 25]. Thanks to this precise assessment we were able to reveal that the volume of testes submitted to 2SLF-SO increased with advancing age, while the difference in volume between the healthy and undescended testis decreased to only 5% at the age of 7–9 years. However, Rosito et al. [26], who evaluated the histology and volume of 44 intra-abdominal testes in 35 patients between 4 months and 14 years old at stages 1 and 2 of the F-S procedure, did not observe significant changes in the volumetric characteristics of the testicles. The vast majority of authors report a significant decrease in TV in cryptorchid patients, which is more evident in testes present in high locations and in patients treated later during childhood [27, 28]. Our study confirmed the lower volume of intra-abdominal testicles, but also shows the possibility of their growth after orchidopexy. TV is an important predictive factor for future testis function in patients with UDT [29].
Most researchers assess the viability of testes and testicular atrophy by palpation [15, 20, 22]. Such a subjective examination allows only an estimated comparison of volumes between the affected and healthy testis. We agree that scrotal US should not be ordered prior to referral to a surgical specialist, but we cannot share the opinion that US and other imaging studies are rarely sensitive diagnostic tools for cryptorchid testes [4]. In our study the findings of diagnostic laparoscopy confirmed the initial US examination results with 100% sensitivity (29/29 gonads) and 100% specificity (6/6 gonads) in diagnostics of non-palpable UDT. Moreover, US is a fast and simple procedure to perform and is the least invasive. It does not scare children, whereas other imaging diagnostic techniques require anesthesia and have the risk of radiation [30].
In 17% of our cases the congenital defect of testicle development (agenesis of testis, vanishing testis) was revealed by laparoscopy. No rudimentary testicular tissue was found. The debate over the management of boys with testicular regression syndrome is still ongoing. The exploration and excision of testicular remnants, as well as blind ending vessels associated with a nubbin, are recommended by many authors, as seminiferous tubules and germ cells may be found in them [31, 32]. However, only single cases of germ cell neoplasia in a testicular remnant are reported in the literature [33]. Nevertheless, undescended testes located intra-abdominally may be dysgenetic gonads or testes in patients with partial androgen insensitivity. The risk of germ cell neoplasia in these gonads is very high [34]. It is recommended that intra-abdominal gonads of high neoplasia risk patients should be removed at the time of diagnosis [7, 35]. However, in our patients there was no suspicion of disorders of sex development.
In conclusion, the two-stage laparoscopic Fowler-Stevens operation is an effective procedure for the treatment of intra-abdominal testicles. Undescended testes get a chance to grow and to have better function after this procedure. The incidence of testicular atrophy is low. However, in our study the number of studied patients within each time point was limited. Greater numbers could allow more reliable statistical comparison.