Introduction
Coronary heart disease (CHD) is the leading cause of morbidity and mortality around the world [1–3]. Stent implantation is broadly applied to patients with either stable angina or acute coronary syndrome [4]. Dual antiplatelet treatment (DAPT) in terms of aspirin plus the P2Y12 receptor antagonist clopidogrel is the standard of care for patients after drug-eluting stent (DES) implantation in the first 12 months [5, 6]. Previous studies have shown that despite adherence to DAPT, a substantial proportion of patients still had occurrence of stent thrombosis, re-infarction and ischemic events [7–9]. The underlying mechanisms are multifactorial and poor platelet response to clopidogrel treatment has been broadly described [10, 11].
Clopidogrel is a pro-drug which requires conversion into its active metabolite to inhibit platelet aggregation [10]. With loss of function (LOF) of the cytochrome P450 2C19 (CYP2C19) allele, the conversion of clopidogrel to its active metabolite in the liver is substantially attenuated, which in turn causes impaired platelet inhibition and increased platelet reactivity [12]. Both randomized controlled trials and prospective cohort studies have also demonstrated a dose-dependent association between CYP2C19 allele dysfunction and increased platelet reactivity and ischemic events [13–15]. Nevertheless, most of these studies were conducted in Caucasian populations, and few prospective studies have been conducted to investigate the effects of CYP2C19 allele variants on clinical outcomes in the Chinese Han population.
We conducted a prospective observational study to evaluate the effects of CYP2C19 allele variants on ischemic and bleeding events in the Chinese Han population after successful coronary artery stenting. The results from our study will not only elucidate the association of CYP2C19 allele variants and clinical outcomes in the Chinese Han population, but could also help to shed light on whether detecting the CYP2C19 allele will be useful to guide antiplatelet treatment and improve clinical outcomes in the future.
MATERIAL AND METHODS
Enrollment of study participants
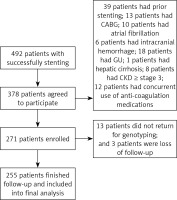
The current study was approved by the Research Ethic Committee of Shenzhen Sun Yat-sen Cardiovascular Hospital, and informed consent was obtained from individual participants after successful stenting. The inclusion criteria were as follows: 18–75 years old, the Han ethnic population, stenting due to stable angina or acute coronary syndrome during the indexed hospitalization, and the first time of coronary artery stenting. The exclusion criteria were as follows: documented history of coronary artery stenting, coronary artery bypass grafting, atrial fibrillation, valve heart disease, intracranial hemorrhage, gastric ulcer, hepatic cirrhosis, hemophilia, aortic dissection or aneurysm, stage 3 or higher of chronic kidney disease (CKD), concurrent use of anti-coagulation medications including warfarin, dabigatran or rivaroxaban, and allergy to clopidogrel or aspirin (Figure 1).
Baseline data collection
Baseline characteristics including demographics (age and gender), smoking status and prior medical history and medication usage were collected by two investigators using a standard questionnaire. Diagnosis of hypertension, dyslipidemia and diabetes mellitus was based on documented history recorded in the medical charts or current usage of relevant medications. Blood pressure (BP) was measured 3 times after participants had been sitting quietly for 10 min (HEM7200, Omron Healthcare, Tokyo, Japan), and the average of the second and third BP readings was calculated and reported [16]. Weight in kilograms was divided by height in squared meters so as to calculate the body mass index (BMI). Venous blood after fasting for 8 h on the second day of hospitalization was obtained and used for complete blood cell count, lipid profile, glycated hemoglobin and creatinine level assessments. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula [17].
Genotyping
Whole blood was obtained during the stenting procedure and was stored in a –80°C refrigerator for genotyping. Genomic DNA was extracted from leukocytes using commercially available kits (Shanghai Baiao Technology Co., Ltd., China) and CYP2C19*2 genotyping was done using amplification refractory mutation system PCR in duplex reaction. Primers were commercially purchased (Shanghai Baiao Technology Co., Ltd., China) and are listed as follows: *2 forward: CAG ACC TTG GCA TATA TGA ATC and *2 reverse: TAT CGG AAG CAG TCA TAT AAG; *2 G specific (sense): ACT ACC ATT GAT TAT ATC CCG and *2 A specific (antisense): GTT ATT TGT TCT GGA TTC CT. All the procedures were conducted in accordance with the Manufacturer’s Manual Instructions (Shanghai Baiao Technology Co., Ltd., China) and the size of the PCR products generated was as follows: CYP2C19*2 forward/reverse of 370 base pair (bp), *2 allele G (normal) of 281 bp and *2 allele A (mutated) of 125 bp.
Platelet reactivity testing
Participants were asked to return to our outpatient clinic for platelet reactivity assessment 4 weeks after coronary artery stenting. Patients were required to not take aspirin and clopidogrel before blood sample drawing. Platelets from a peripheral vein were stimulated with adenosine diphosphate (10 μmol/l) and the absolute reduction in maximal platelet aggregation from baseline (ΔMPA) was reported and compared between different genotype groups.
Follow-up and clinical outcomes
Participants were followed up for 6 months after indexed stent implantation at an outpatient clinic. Ischemic events included definite stent thrombosis diagnosed by angiography, ischemic stroke, myocardial infarction and sudden cardiac death; and bleeding events included intracranial hemorrhage and gastrointestinal bleeding. Both ischemic and bleeding events were adjudicated by two independent cardiologists who did not participate in the current study and also were not informed of the genotypes of each participant. On-treatment medications at the follow-up visit were also obtained.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile ranges), and categorical variables were expressed as number and frequency of cases. Between-group differences were evaluated by the independent Student t test or the Mann-Whitney U test for continuous variables as appropriate, or the χ2 analysis or Fisher’s exact test for categorical variables as appropriate. Cox proportional hazards regression analysis was used to evaluate the predictive value of the CYP2C19*2 allele for ischemic and bleeding events, respectively. The hazard ratio (HR) represents the risk associated with 1 reduced-function allele of CYP2C19*2 for ischemic and bleeding events. Sensitivity analyses were performed to evaluate whether diabetes mellitus (presence versus absence), usage of proton pump inhibitor (yes versus no) and indications for coronary artery stenting (stable angina versus acute coronary syndrome) were associated with ischemic and bleeding events. Statistical analysis was conducted in SPSS 23.0 (IBM, USA). All p-values were 2 sided, and statistical significance was defined as p < 0.05.
RESULTS
Baseline characteristics
Between July 2016 and April 2017, a total of 492 patients successfully underwent stenting in our hospital and 378 patients agreed to participate in the current study. After exclusion of 107 patients, 271 patients were enrolled. Thirteen patients did not return for genotyping and 3 patients were lost to follow-up. No significant difference in the baseline characteristics between the 16 patients and the 255 patients who were included in the final analysis was observed.
As presented in Table I, the mean age was 57.5 years and male participants accounted for 57.3%, and 57.7% and 42.3% of participants presented with stable angina and acute coronary syndrome, respectively. Before the index hospitalization, 74.1% and 18.0% of participants were being treated with aspirin and clopidogrel, respectively, outside of the hospital. No significant differences in baseline characteristics were observed between different CYP2C19*2 genotype groups except the patients with AA genotype were more likely to be male.
Table I
Baseline characteristics comparison
Genotype distribution of CYP2C19*2
The genotype frequencies for each allele were consistent with Hardy-Weinberg equilibrium. In current enrolled Chinese Han participants, the prevalence of the homozygous (AA) and heterozygous (GA) CYP2C19*2 variants was 3.5% and 24.7%, respectively, and the prevalence of the wild type genotype (GG) was 71.8%.
Effects of CYP2C19*2 variant on platelet reactivity
Four weeks after stenting, platelet reactivity expressed as ΔMPA was compared between different CYP2C19*2 genotypes. Compared to the GG and GA genotype groups, the ΔMPA was significantly lower in the AA genotype group (GG 43.6 ±7.8%, GA 31.9 ±6.5%, and AA 24.8 ±5.3%, p < 0.01 for trend). The difference in the ΔMPA between the GG and GA genotype groups was also statistically significant (p = 0.028).
Comparisons of clinical characteristics after 6 months’ follow-up
As presented in Table II, no significant differences in risk factors, medications usage, numbers of stents implanted and site of coronary artery lesions were observed between different genotype groups, except for a significantly higher percentage of patients in the GG genotype group taking proton pump inhibitors during follow-up. Compared to the clinical characteristics at baseline, the percentages of current smokers were significantly reduced, while the percentages of patients with anti-hypertensive, anti-diabetic, anti-platelet, and statin treatment were significantly increased in all three genotype groups.
Table II
Comparisons of clinical characteristics after 6 months’ follow-up
| Variables | GG (n = 183) | GA (n = 63) | AA (n = 9) | P-value |
|---|---|---|---|---|
| Current smoker, n (%) | 37 (20.2) | 10 (15.9) | 2 (22.2) | 0.061 |
| Systolic BP [mm Hg] | 131 ±17 | 129 ±18 | 127 ±13 | 0.174 |
| Diastolic BP [mm Hg] | 72 ±13 | 70 ±15 | 69 ±11 | 0.205 |
| Heart rate [beat per minute] | 73 ±13 | 75 ±14 | 71 ±10 | 0.158 |
| Hemoglobin [g/l] | 13.5 ±1.2 | 13.8 ±1.4 | 13.6 ±1.5 | 0.192 |
| Platelet [× 109/l] | 182 ±41 | 186 ±49 | 193 ±46 | 0.133 |
| Glycated hemoglobin (%) | 6.4 ±1.3 | 6.2 ±1.4 | 6.3 ±1.1 | 0.254 |
| Total cholesterol [mmol/l] | 4.9 ±0.8 | 4.8 ±0.7 | 4.8 ±0.9 | 0.140 |
| Triglyceride [mmol/l]* | 1.8 (0.8–2.8) | 1.8 (0.7–3.2) | 1.7 (0.8–2.9) | 0.302 |
| Creatinine [μmol/l] | 75.2 ±14.3 | 76.6 ±13.7 | 74.4 ±12.9 | 0.115 |
| eGFR [ml/min/1.73 m2] | 81.3 ±15.4 | 82.8 ±13.2 | 83.9 ±14.6 | 0.224 |
| Aspirin, n (%) | 183 (100) | 63 (100) | 9 (100) | 1 |
| Clopidogrel, n (%) | 183 (100) | 63 (100) | 9 (100) | 1 |
| Statins, n (%) | 183 (100) | 63 (100) | 9 (100) | 1 |
| Anti-hypertension, n (%) | 122 (66.7) | 41 (65.1) | 6 (66.7) | 0.104 |
| Anti-diabetes, n (%) | 72 (39.3) | 26 (41.3) | 4 (44.4) | 0.172 |
| Proton pump inhibitor, n (%) | 32 (17.5) | 9 (14.3) | 1 (11.1) | 0.016 |
| Number of stents | 1.9 ±0.5 | 1.8 ±0.6 | 1.6 ±0.5 | 0.260 |
| LAD stenting, n (%) | 121 (66.1) | 42 (66.7) | 6 (66.7) | 0.131 |
| LCX stenting, n (%) | 37 (20.2) | 14 (22.2) | 2 (22.2) | 0.259 |
| RCA stenting, n (%) | 84 (45.9) | 29 (46.0) | 4 (44.4) | 0.207 |
| LM stenting, n (%) | 4 (2.2) | 1 (1.6) | 0 | 0.164 |
| Firebird, n (%) | 70 (38.3) | 24 (38.1) | 3 (33.3) | 0.085 |
| Excel, n (%) | 31 (16.9) | 10 (15.9) | 1 (11.1) | 0.116 |
| Taxus, n (%) | 36 (19.7) | 13 (20.6) | 2 (22.2) | 0.184 |
| Enderver, n (%) | 46 (25.1) | 16 (25.4) | 3 (33.3) | 0.072 |
Effects of CYP2C19*2 variant on incidence of ischemic events
After 6 months’ follow-up, 3.3%, 4.8% and 11.1% of patients experienced ischemic events in the GG, GA and AA genotype groups, respectively (p = 0.003 for trend, Table III). Specifically, 2 and 4 patients in the GG genotype group had stent thrombosis and ischemic stroke, respectively; 1 and 2 patients in the GA genotype group had stent thrombosis and myocardial infarction, respectively; and 1 patient in the AA genotype group had stent thrombosis. Using Cox proportional hazards regression analysis, after adjustment for age, male gender, smoking, hypertension, diabetes mellitus, dyslipidemia, eGFR, number of stents implanted, site of stent implantation, type of stent implantation and medications usage during follow-up, the AA genotype was significantly associated with ischemic events, with hazard ratio (HR) 1.19 and 95% confidence interval (CI) 1.08–1.30 (p = 0.013). Compared to the GA genotype group, the AA genotype was marginally associated with ischemic events, with HR = 1.08 and 95% CI: 0.96–1.19 (p = 0.058).
Effects of CYP2C19*2 variant on incidence of bleeding events
After 6 months’ follow-up, 2.2%, 1.6% and 0% of patients experienced bleeding events in the GG, GA and AA genotype groups (p = 0.153 for trend, Table III). Specifically, 4 patients in the GG genotype group had gastrointestinal bleeding; 1 patient in the GA genotype group had gastrointestinal bleeding. Using Cox proportional hazards regression analysis, after adjustment for age, male gender, smoking, hypertension, diabetes mellitus, dyslipidemia, eGFR, number of stents implanted, site of stent implantation, type of stent implantation and medications usage during follow-up, the GG genotype was not independently associated with bleeding events, with HR of 1.04 (95% CI: 0.92–1.13, p = 0.106) compared to the AA genotype group.
Sensitivity analysis
Sensitivity analysis was performed and the results indicated that presence of diabetes was marginally associated with increase of both ischemic and bleeding events, with HR of 1.19 (95% CI: 0.98–1.27, p = 0.054) and 1.16 (95% CI: 0.96–1.22, p = 0.061), respectively, while indications for stenting and usage of proton pump inhibitors at baseline did not show any significant association with either bleeding or ischemic events, respectively.
Discussion
Our present study shows that in the Chinese Han population, more than one fourth of participants had either the homozygous (AA) or the heterozygous (GA) CYP2C19*2 allele variant. Compared to the wild type genotype (GG), participants with presence of the A variant allele had significantly higher platelet reactivity despite adherence to aspirin and clopidogrel treatment. After 6 months’ follow-up, compared to the wild type genotype, the presence of the A variant allele was independently associated with documented ischemic events in both the homozygous and heterozygous genotype groups after adjustment for traditional risk factors and on-treatment medications, while no significantly increased bleeding risk was observed in the wild type genotype group compared to the other two groups.
Numerous genotyping studies have been conducted in the Caucasian populations in the last decade. Both the randomized controlled trial and prospective cohort studies have demonstrated the clinical validity and utility of genotyping the CYP2C19*2 allele for guiding antiplatelet treatment. For example, Mega et al. [13] reported that among patients treated with clopidogrel, carriers of a reduced-function CYP2C19 allele had significantly lower levels of the active metabolite of clopidogrel, impaired platelet inhibition, and a 3-fold increased risk of stent thrombosis compared to the non-carriers. In a cohort study including 259 young patients (aged < 45 years) who survived a first myocardial infarction and were exposed to clopidogrel treatment for at least a month, Collet et al. [14] found that the CYP2C19*2 genetic variant was associated with 4-fold higher risk of cardiovascular events even after adjusting for traditional risk factors. Trenk et al. [18] also observed that in patients undergoing percutaneous coronary intervention (PCI), those carrying at least one CYP2C19*2 allele were more likely to have high on-clopidogrel platelet reactivity and a poor clinical outcome after coronary stent placement after 1 year’s follow-up. Consistent with prior findings, we also observed that in the Chinese Han population, the presence of the variant allele of CYP2C19*2 had poorer platelet inhibition and more ischemic events after 6 months’ follow-up. In addition, dose-dependent associations of CYP2C19*2 variant allele and platelet reactivity and incidence of ischemic events were also observed, which were strengthened by excellent adherence to antiplatelet treatment. Compared to the prior studies conducted in Chinese populations, our current study not only evaluated the effects of the CYP2C19*2 variant on ischemic events but also investigated whether this variant is associated with bleeding events. Also our current studied showed that patients with presence of the A variant allele had significantly higher platelet reactivity and more ischemic events but without a significant difference in bleeding events.
Recently, Cavallari et al. [19] reported that the risk for major adverse cardiovascular events was significantly higher in patients with a loss-of-function allele prescribed clopidogrel versus alternative therapy such as prasugrel or ticagrelor. However, no difference in major adverse cardiovascular events between patients without a loss-of-function allele and loss-of-function allele carriers prescribed alternative therapy was observed. They concluded that the real-world investigation demonstrated a higher risk for cardiovascular events in patients with a CYP2C19 loss-of-function allele if clopidogrel versus alternative therapy was prescribed. A prior randomized controlled trial also showed that personalized antiplatelet therapy according to CYP2C19 genotype after PCI could significantly decrease the incidence of major adverse cardiovascular events and the risk of 180-day stent thrombosis in a Chinese population [20]. In this previous study, the investigators applied the strategy of increasing maintenance clopidogrel doses plus cilostazol to overcome loss of function of the CYP2C19 allele. It is interesting and important to evaluate whether the alternative therapy could also reduce ischemic events but will not increase bleeding events in the Chinese Han population in the future.
With respect to the potential ethnic/race difference in bleeding risk with antiplatelet treatment [21–25], we also compared the incidence of bleeding events between different genotype groups. Although a slightly higher incidence of bleeding events was observed in the wild phenotype compared to the loss-of-function genotype (2.2% vs. 0%), no independent association of GG phenotype and bleeding risk was observed in the regression model. We also performed sensitivity analysis to explore whether presence of diabetes, indication for PCI and usage of proton pump inhibitors at baseline were associated with ischemic and bleeding events. Presence of diabetes was marginally associated with increase of both ischemic and bleeding events, while indications for stenting and usage of proton pump inhibitors at baseline did not show any significant association with either bleeding or ischemic events, respectively. Since our current study was compromised by the relatively small sample size, few bleeding events during follow-up, and the participants with diabetes and usage with proton pump inhibitors at baseline were few, future studies with larger sample size and longer follow-up duration are required to confirm or refute these findings.
The clinical implications of our current study were two-sided: on the one hand, in the future, building on our current findings, we can design randomized clinical trials to evaluate whether CYP2C19 guided anti-platelet selections can reduce ischemic events as well as avoid unwanted bleeding side effects. On the other hand, our current study provided additional knowledge of the role of CYP2C19 variants in cardiovascular outcomes in the Chinese Han population, which might help physicians to select appropriate antiplatelet medications for patients after coronary artery stenting.
Our study was strengthened by its prospective design, excellent adherence to antiplatelet therapy and low rate of loss to follow-up. However, some limitations also deserve to be mentioned. Firstly, the relatively small sample size did not allow us to perform subgroup analysis, and the sensitivity analysis was also somewhat underpowered. Secondly, the short-term follow-up did not allow us to observe more events, which may influence our interpretation of the association of the CYP2C19*2 allele variant and clinical outcomes. Thirdly, although we extensively adjusted for potential covariates, residual confounding factors could still exist and elicit biases. Last but not least, the current study was conducted in the Chinese Han population and findings from the current study should not be extrapolated to other ethnic groups.
To our knowledge, the future direction should focus on whether CYP2C19 guided antiplatelet treatment can help reduce cardiovascular outcomes in the Chinese Han population. In addition, whether switching from clopidogrel to novel antiplatelet medications such as ticagrelor based on CYP2C19 variants can also improve outcomes should also be a clinically relevant direction.
In conclusion, our current study indicates that genotyping of CYP2C19*2 may be useful to guide antiplatelet treatment in the Chinese Han population after successful stenting. Future randomized controlled trials are warranted to investigate whether genotype-guided antiplatelet treatment could improve antiplatelet treatment and reduce cardiovascular outcomes.