Introduction
The embryo transfer (ET) strategy is one of the most crucial steps in in-vitro fertilization (IVF) protocols. There are two commonly used ET protocols: fresh and frozen [1]. On the other hand, ET may be performed on the third day or fifth day of fertilization. Day 3 embryo (D3E) or day 5 embryo (blastocyst) (D5E) transfer can be prefered [2]. Recently, elective single embryo transfer (eSET) has become more popular to achieve higher singleton pregnancy rates (PR) and reduce the multiple gestations [1]. eSET may allow the IVF centers to retrieve the most successful ET from the pool of embryos. Therefore eSET is the most effective strategy to minimize multiple pregnancies, which are associated with increased abortion rates (AR), as well as increases in maternal/neonatal morbidity and mortality [1].
Especially frozen-thawed eSET has better pregnancy outcomes as compared to fresh eSET [3, 4]. Enhanced laboratory techniques and increased survival rates of frozen-thawed embryos by vitrification have resulted in a trend toward favoring the frozen-thawed ET as a preferred transfer strategy. Additionally, frozen-thawed ET may provide a more natural endometrial environment and endometrial receptivity without elevated levels of hormones which may adversely affect implantation [5].
In the frozen-thawed ET strategy, there have been several endometrial preparation protocols including true natural cycle, modified natural cycle, artificial cycle with or without suppression and mild ovarian stimulation [6]. All these procedures result in similar PR [6, 7]. Although there is no superiority of any endometrial preparation techniques for frozen-thawed ET, because it is more practical, the artificial cycle is the preferred one at IVF centers [5].
The most common problem in IVF cycles is recurrent implantation failure (RIF), seen in 10% of infertile patients [8]. The current definition of RIF is failure to achieve pregnancy following three or more IVF/intracytoplasmic sperm injection (ICSI) treatments with one or two high quality embryos or more than 10 high quality embryos transferred in fresh or frozen cycles.
There is extensive literature regarding treatment strategies for RIF including GM-CSF, peripheral blood mononuclear cell (PBMC) intrauterine insemination (IUI), hCG or LIF, endometrial injury in previous cycles or in the follicular phase of ongoing cycles. All of these strategies have positive immunomodulator effects on implantation [9].
Our prospective randomized study is the first trial in the literature to evaluate the effects of IUI before frozen-thawed elective single embryo transfer (FT-eSET) in unexplained subfertility patients undergoing IVF treatment.
Material and methods
This prospective randomized study was conducted between October 2017 and June 2018 with the approval of the Ethics Committee of Erzincan University (2017-42). The population of the study was composed of 200 unexplained subfertility patients who underwent frozen-thawed eSET. These 200 patients were randomized into two groups in a ratio of 1 : 1 by means of computer-generated random numbers (http://www.randomization.com). The IUI preceding FT-eSET group was composed of 100 patients who underwent IUI before 6 days of FT-eSET.
Couples were diagnosed with unexplained subfertility based on a history of regular ovulation in women below the age of 35, normal sperm analysis, normal hysterosalpingography findings and no pelvic disease (endometriosis or pelvic inflammatory disease) [10]. Patients who had three or more previous unsuccessful IVF cycles, with hereditary thrombophilia or an abnormality in the thrombophilia tests were not included in the study.
In our clinical IVF strategy, the best quality blastocysts, at least 4AA, were frozen to increase implantation rates in normoresponder women without the presence of endometrial polyps, myoma or hydrosalpinx.
The age, body mass index, previous IVF cycles, baseline FSH, AMH, number of oocytes retrieved, average number of metaphase II oocytes, 2PN fertilization rate, and number of frozen blastocysts were noted. The main outcome measures of the study were conception rate, implantation rate, biochemical miscarriage, clinical pregnancy loss, ongoing pregnancy and live birth.
Artificial endometrium preparation was done with a fixed dose of 2 mg oral estradiol tablets, three times a day (total 6 mg) (Estrofem, Novo Nordisk, Denmark). Oral estrogen was administered starting from the second day of the menstrual cycle after basal ultrasonography to rule out the presence of ovarian cysts or other pelvic pathologies.
USG scan was repeated on day 10 of the cycle to monitor endometrial thickness and to observe whether there was a spontaneously growing dominant follicle. If there was a dominant follicle or the endometrial thickness was 7 mm or lower, the cycle was cancelled. However, no such event occurred. On day 15, a further USG scan was performed and patients were started on progesterone vaginal gel (Crinone 8%; Merck Serono, Switzerland) twice a day. Frozen-thawed embryo transfer was performed on the 5th day of progesterone administration. On the morning of the day of transfer, vitrified blastocysts were thawed and cultured in the culture medium (Life Global, Brussels, Belgium), supplemented with 10% Plasmanate (Life Global, Brussels, Belgium) for 3 to 4 h to observe re-expansion and viability.
Good or top-quality blastocysts (at least 4AA) were vitrified on day 5 with Kitazato vitrification media according to the manufacturer’s instructions, using Cryotops as carriers. Blastocysts were thawed with Kitazato warming medium according to the manufacturer’s instructions. Embryos were first checked for survival 30 min after thawing.
In the IUI preceding FT-eSET group, IUI was timed 6 days before FT-eSET: a day before starting the administration of progesterone vaginal gel. For intrauterine insemination, the double gradient method was used for semen preparation. The sperm sample was collected by masturbation after 3–5 days of abstinence. The collected samples were then kept in an incubator at 37°C for 20 min for liquefaction. After liquefaction, samples were examined for the sperm count, motility and morphology. They were then centrifuged with double-layer (upper 1 ml 45%, lower 1 ml 90% Pure Sperm) gradient medium, after which the filtrate was collected and washed. Samples were washed twice with sperm washing medium and during the washing process, centrifugation was performed at 1,600 rpm for 10 min. Supernatant was carefully removed and layered with 0.7 ml of G-sperm and kept in the incubator at 37°C, tilted at an angle of 45 degrees for 20 min. After 20 min, 0.5 ml of the upper layer, expected to contain highly motile spermatozoa, was gently aspirated using a soft catheter (Genetics). A total of 1 ml of sperm preparation was slowly injected into the uterus, 6 days before FT-eSET.
Statistical analysis
Numerical variables were presented as means ± SD. Non-normally distributed metric variables were analyzed by the Kruskal-Wallis test and Mann-Whitney U test. After the confirmation of normal distribution, paired t tests were used to compare the values of the groups. All other analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). P ≤ 0.05 was considered statistically significant.
Results
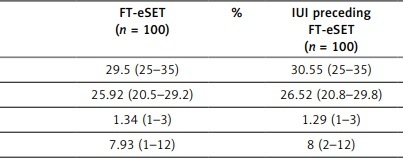
In the study, both groups were comparable in baseline demographics. Previous IVF cycles, baseline FSH and AMH levels were similar between the two groups. Additionally, number of oocytes retrieved, pronuclear stage embryos (PN2) and the number of frozen blastocysts were also similar. The IUI preceding FT-eSET group was associated with higher rates of clinical pregnancy, 54% vs. 42%, but not significantly. Similarly, IR, PR and live birth rate were higher in the IUI preceding FT-eSET group but not significantly. In the IUI preceding FT-eSET group, the biochemical and clinical abortion rates were lower than in the control group (9.5% vs. 14.2% and 5.5% vs. 5.2%, respectively). However, no statistically significant difference was found between the two groups, either (Table I, p = 0.7 and p = 0.57).
Table I
Comparison of the characteristics and results of two groups
Discussion
In this prospective randomized trial, we firstly investigated the effect of IUI before FT-eSET with artificial endometrial preparation, in patients with unexplained subfertility. Our results revealed that IUI preceding FT-eSET may increase PR and decrease AR. The prostaglandins, cytokines, and growth factors in semen may increase endometrial receptivity [10]. In particular, the vascular endothelial growth factor in the human spermatozoa is pivotal for embryo implantation [10, 11]. Additionally, the movements of sperm may mimic the scratching effect on endometrium which increases implantation by inflammatory and immunologic mechanisms [12]. Therefore, our results support the positive effects of sperm on implantation.
Geva et al. showed that IUI with ovulation induction might improve PR in FT-ET [10]. In contrast to our study, 2PN and up to 8-cell stage embryos were transferred, IUI with ovulation induction was preferred and more than one embryo were transferred. According to this study, PR was increased, multiple pregnancies were decreased, and cancellation rates in endometrial preparation cycles were decreased. In our study we chose artificial endometrial preparation instead of ovulation induction and transferred a single D5 embryo, in order to observe the effect of IUI on the main outcome measures, in itself.
In regards to the practice of implantation improvement techniques in RIF, there is a wide range of literature including GM-CSF, peripheral blood mononuclear cell, IUI, hCG or LIF, endometrial injury in previous cycles or at the follicular phase of ongoing cycles [8, 9, 13]. There is no additional advantage to either one. All these procedures claimed that the immunomodulatory effects on endometrium increased PR. Additionally, in a recent study, intrauterine semen administration had regulatory effects on T lymphocytes, which have the most important role in implantation [14]. But in our study, insemination during the FET cycle is more natural and mimics the spontaneous pregnancy. According to our hypothesis, sperm has not just an immunomodulation effect on endometrium but also has physical effects that simulate scratching.
Although the effects of endometrial injury with the IUI catheter during the IVF cycle on PR are conflicting, there is an interesting study showing an improvement of PR in IUI cycles, solely [15]. Similarly, according to the last Cochrane review [16] endometrial injury performed between day 7 of the previous cycle and day 7 of the embryo transfer (ET) cycle is associated with an improvement in live birth and clinical pregnancy rates in women with RIF. Additionally, there are no proven detrimental effects of endometrial injury including miscarriage, multiple pregnancies or bleeding. In our study, although not statistically significant, IR and PR were higher and AR was lower in IUI preceding FT-eSET. However, if the number of patients were increased, we would obtain a statistically significant difference in IR. So, large studies may be needed to confirm our results.
Another limitation of the study is in regards to a claim that sperms exert physical effects that stimulate scratching. We preferred IUI in which sperms just swim within a liquid medium. In a future study, we can apply ejaculate into the uterine cavity and examine the effect of ejaculate on endometrium. Furthermore, larger series of this study will clearly appear and confirm the IUI effect on implantation.
In conclusion, this is the first prospective randomized study indicating that IUI before FT-eSET can be chosen to improve PR in couples with unexplained infertility. The PR has been improved by combining IUI and FT-ET with ovulation induction. Performing IUI before FT-eSET prevents biochemical abortions. Further large randomized controlled trials need to be performed to clarify our results.