Introduction
The bacterium Helicobacter pylori causes gastroduodenal inflammation [1]. H. pylori infection is present in 28–84% of populations around the world [2]. The precancerous lesions of chronic gastritis are well known to be caused by H. pylori infection [3–5]. H. pylori eradication using a regimen containing anti-bacterial agents is useful not only for healing gastroduodenal ulcers but also for preventing gastric cancer [6].
C-reactive protein (CRP), produced in the liver and released into blood, is a representative marker reflecting systemic inflammation [7]. This protein is increased not only in acute but also in chronic diseases, and its slight change in chronic diseases at a low-grade subclinical level has pathophysiological relevance [7–9]. In H. pylori infection, a low-grade chronic increase in circulating inflammatory markers, especially CRP, is observed [10]. Such chronic inflammation is also known to initiate and exacerbate various systemic pathologies [11]. H. pylori infection is indicatively related to other gastric and even extra-gastric diseases, including gastric mucosa-associated lymphoid tissue lymphoma, functional dyspepsia, idiopathic thrombocytopenic purpura, iron deficiency anemia, chronic urticaria, metabolic disorder, ischemic heart disease, cognitive impairment, and neurodegeneration [6, 12–16].
The clinical manifestations of some diseases may be improved after H. pylori eradication in relation to the CRP levels [4–6, 17, 18]. As the CRP levels are typically low in such inflammatory states, changes in them may be overlooked. The effect of H. pylori eradication on CRP remains undetermined; accordingly, the present study was designed to evaluate the effect of H. pylori eradication on circulating CRP levels through a meta-analysis.
Material and methods
The relevant literature was searched using the PubMed database from its inception to June 3, 2020. The following keywords were used to search: (“Helicobacter pylori”[Mesh] OR “Helicobacter pylori”[tiab] OR “H. pylori”[tiab]) AND (“C-Reactive Protein”[Mesh] OR “c-reactive protein”[tiab] OR “CRP”[tiab]) AND (“eradicat*”[tiab]). Studies that focused on the effect of H. pylori eradication on CRP were included. Non-English-language and non-adult studies were excluded.
First, all candidate articles searched were independently screened according to the title and abstract. Original articles that did not focus on H. pylori and CRP were excluded. Second, the full text of potentially relevant abstracts was evaluated for eligibility. Original articles that did not focus on H. pylori and CRP were further excluded. When the researchers’ opinions matched, the articles were considered eligible. The agreement between the two researchers was determined by the κ value. The eligibility was judged based on the two researchers’ opinions. Third, summary tables of the respective articles were extracted and created.
The risk of bias was independently assessed using Risk of Bias (RoB) 2 [19] for randomized studies and the Newcastle-Ottawa Quality Assessment Scale (NOS) [20] for non-randomized studies. RoB 2 was assessed as described in the Cochrane handbook for the following six domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Each domain was classified into one of three categories (high risk, low risk, some concerns). The NOS assigns a maximum of 9 points to studies of the highest quality according to three quality parameters: selection, comparability and outcome; high score means better quality. Any disagreement of the assessment by the two researchers was resolved by discussion.
The standardized mean difference (SMD) with the 95% confidence interval (CI) of the mean change in the CRP levels after H. pylori eradication was calculated as described in the Cochrane handbook [21]. The meta-analysis was performed by comparing each individual case before and after eradication, regardless of study design, because the CRP levels differ among individuals. Similar analyses were performed in earlier studies [22–24]. The statistical heterogeneity was evaluated by visual inspection of the forest plots and calculating the I2 statistic (I2 values of 0–40%: might not be important; 30–60%: may represent moderate heterogeneity; 50–90%: may represent substantial heterogeneity; 75–100%: may represent considerable heterogeneity) [21]. When there was substantial heterogeneity (I2 > 50%), the potential reason for the heterogeneity was assessed using a subgroup analysis. The potential publication bias was assessed by visual inspection of the funnel plot [21]. The meta-analysis was performed with a random-effects model using the genetic inverse method in the Review Manager 5.4 software program of RevMan 2020.
Results
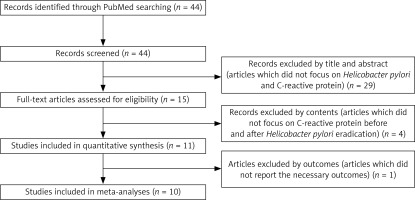
Figure 1 shows the process of selection of articles that reported the effect of H. pylori eradication on CRP. After the first step, a total of 44 records were searched, and 15 articles were considered candidates (inter-reader agreement, κ = 0.894). At the second step, four articles were excluded because these articles did not meet the criteria. Eleven studies were then identified in quantitative synthesis (inter-reader agreement, κ = 1.0) [25–35]. Finally, 10 studies (total of 642 subjects) were meta-analyzed, as 1 article [30] did not report important measures, such as the standard deviation, range, or standard error of the CRP levels.
Table I shows the summary of the reviewed studies. Four studies were reported from the Middle East (Turkey [27], Iran [32], Egypt [33, 35]), 4 from Europe (Spain [25, 34], Italy [26], the Netherlands [29]), 1 from the United States [31] and 1 from Korea [28]. The study design consisted of 2 randomized controlled trials (RCTs), 2 cohort studies, 2 case-controlled studies and 4 before-after studies. The age reported in the reviewed studies was 49.9 (median (range: 44.7–56.0)) years. The CRP level was 6.0 (median (range: 0.0028–310)) mg/l before H. pylori eradication and 5.8 (median (range: 0.0026–100)) mg/l after it. The periods of eradication ranged from 7 to 14 days. The H. pylori eradication rate was 96.4% (median (range: 73.3–100%)). The follow-up period, meaning the interval period to examined CRP levels before and after eradication, was 3 (median (range: 1.5–24)) months.
Table I
Summary of characteristics of the reviewed studies
| Authors [ref no.] | Year | Design | Country | Comorbidities | Subject no. | Age [years] | Therapy [days] | Follow-up [months] | Eradication rate (%) | C-reactive protein [mg/l] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before eradication | After eradication | ||||||||||
| De Luis [25] | 1999 | Before-after study | Spain | Type 1 diabetes mellitus | 22 | 45.1 | 10 | 3 | 73.3 | 4.8 ±0.7 | 3.3 ±0.4 |
| Zentilin [26] | 2002 | Before-after study | Italy | Rheumatoid arthritis | 28 | 56 | 7 | 24 | 96.4 | 310 (180–1220)a | 100 (30–400)a |
| Kanbay [27] | 2005 | Before-after study | Turkey | – | 57 | 49.9 | 14 | 2 | 100 | 85 ±28 | 77 ±27 |
| Park [28] | 2005 | Case-control study | Korea | – | 29 | 44.7 | 7 | 12 | 100 | – | 0.2 ±1.3b |
| Steen [29] | 2009 | Randomized controlled trial | Netherlands | Rheumatoid arthritis | 55 | – | – | 12 | – | 6.0 (2.7–16)b | 5.8 (2.5–9.3)a |
| Siddiqui [31] | 2009 | Before-after study | USA | – | 46 | 55.8 | 14 | 2 | 93.5 | 30 ±29 | 11.4 ±14.2 |
| Kachuei [32] | 2016 | Randomized controlled trial | Iran | Prediabetes | 26 | 52.3 | 14 | 1.5 | 88.5 | 1.1 ±0.2 | 1.1 ±0.2 |
| Abdel-Razik [33] | 2018 | Cohort study | Egypt | Non-alcoholic fatty liver disease | 127 | 49.8 | 14 | 3 | 78.9 | 0.0031 ±0.002 | 0.0026 ±0.002 |
| Cornejo-Pareja [34] | 2019 | Case-control study | Spain | Healthy | 40 | 47.0 | 10 | 3 | 97.5 | 41 ±4 | 36 ±3 |
| Abdel-Razik [35] | 2020 | Cohort study | Iran | Cirrhosis | 212 | 53 | 14 | 3 | 78.5 | 0.0028 ±0.002 | 0.0028 ±0.005 |
Tables II and III show the quality scores for the reviewed randomized and non-randomized studies, respectively. The overall risks of bias in 2 RCTs were classified into some concerns due to unclear random sequence generation and reporting bias. The quality assessment of 8 non-RCTs were moderate scores in the range of 5–6. Regarding publication bias, the funnel plot appeared to be asymmetric, with some missingness at the lower right portion of the plot, suggesting possible publication bias (Supplementary Figure S1).
Table II
Quality scores for the reviewed randomized studies
| Authors [ref no.] | Risk of bias 2 tool assessment | |||||
|---|---|---|---|---|---|---|
| Bias arising from the randomization process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported results | Overall risk of bias | |
| Steen [29] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Kachuei [32] | Some concerns | Low | Some concerns | Low | Some concerns | Some concerns |
Table III
Quality scores for the reviewed non-randomized studies
| Authors [ref no.] | Newcastle-Ottawa Quality Assessment Scale | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Total | ||||||
| Representatives of the exposed cohort/adequate case definition (0, 1) | Selection of the non-exposed cohort/representative of cases (0, 1) | Ascertainment of exposure/ selection of controls (0, 1) | Demonstration that outcome of interest was not present at start of study/definition of controls (0,1) | Comparability on the basis of design or analysis (0, 1, 2) | Assessment of outcome/ exposure (0, 1) | Was follow-up long enough for outcomes to occur? (0, 1) | Adequacy of follow-up of cohorts (0, 1) | Score | |
| De Luis [25] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Zentilin [26] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Kanbay [27] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Park [28] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 5 |
| Siddiqui [31] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Abdel-Razik [33] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Cornejo-Pareja [34] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Abdel-Razik [35] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
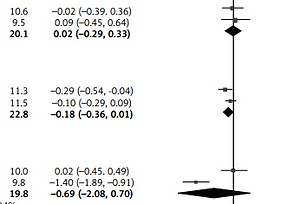
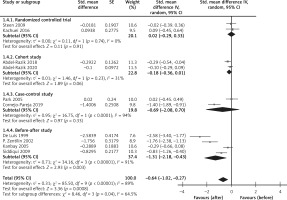
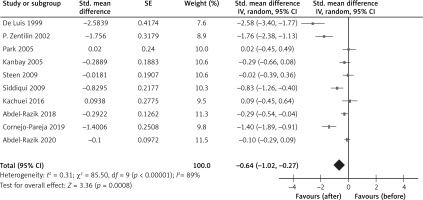
The CRP levels were reduced after H. pylori eradication not only in healthy subjects [34] but also in subjects with diabetes mellitus [25], rheumatoid arthritis [26], non-alcoholic hepatitis [33], and cirrhosis [35]. From the results of the overall meta-analysis, H. pylori eradication was associated with reduced CRP levels (SMD = –0.64; 95% CI: –1.02 to –0.27; I2 = 89%, Figure 2). In a subgroup analysis by study designs, the reduction of CRP in the eradicated patients was not found in the group of RCTs, while it was found in the group of before-after studies (Figure 3).
Figure 2
Forest plot of the change in the C-reactive protein level after Helicobacter pylori eradication
In additional subgroup analysis by comorbidity (such as glucose intolerance/diabetes, rheumatoid arthritis, chronic liver disease), H. pylori eradication reduced the CRP levels in both patients with and without comorbidities (Supplementary Figure S2). In a subgroup analysis by country (which can reflect ethnicity), H. pylori eradication reduced the CRP levels in patients in Europe and the Middle East, while this seemed to be an insufficient analysis because there was only one report in the United States or Asia (Korea) (Supplementary Figure S3). In a subgroup analysis by the follow-up period, H. pylori eradication reduced the CRP levels more in patients with ≥ 3-month periods relative to those with < 3-month periods (Supplementary Figure S4).
Discussion
The overall meta-analysis revealed that H. pylori eradication was associated with reduced CRP levels. However, the result was not similarly supported by a subgroup of the RCTs, which meant that the evidence as regards the effects of H. pylori eradication on the reduction of CRP levels was weak. As a systemically low-grade inflammation, as expressed by the CRP level, is related to the development and progression of various diseases [11], the present study findings would promote our understanding of the pathophysiology.
Although the present study does not mention the etiological mechanisms responsible for the relationship between CRP and H. pylori eradication, several potential factors involved have been assumed. The mucosal response to H. pylori infection induces the production of cytokines (e.g. interleukin-6, tumor necrosis factor-γ) on CRP, mainly in the liver hepatocytes [36, 37]. H. pylori eradication suppresses the cytokine production, which can reduce the CRP level [38]. The microbiome is a possible contributor as the diverse microbiome is thought to modulate inflammation and the host’s immune system favorably [39–41]. A microbiome lacking in the diversity due to infection can induce metabolic endotoxemia with an increased CRP level [40, 41]. The anti-biotic therapies against H. pylori infection alter the microbiome, leading to a reduction in H. pylori-induced inflammation [42, 43].
Furthermore, the clinical manifestations of some chronic diseases may be improved in association with the reduced CRP levels [17, 18]. While it is not fully clarified whether CRP is the etiological agent of inflammation or a simple indicator/marker related to inflammation [11, 44], the pathophysiology of chronic diseases appears to be generally improved in a relatively long period. When the subanalysis by the follow-up period of eradicated patients was conducted, the CRP levels were more reduced in the patients with a longer follow-up period (as shown in (Supplementary Figure S4). Future work on the relationship of CRP and clinical manifestations with the period after H. pylori eradication may shed light on the etiological mechanisms.
The present study had some strengths. First, the CRP levels were analyzed before and after H. pylori eradication among the same individuals. Accordingly, the individual characteristics were not likely to have had a large effect on the results. Second, the duplicate assessments were performed for the eligibility, risk of bias and data abstraction in the review process.
There were some study limitations. First, circulating CRP levels can be associated with age, gender, ethnicity, lifestyle and comorbidity [45], although few within-person variations of CRP are revealed at the year-to-year level [46]. The subanalysis by comorbidity and country did not reveal a clear difference in the results (as shown in (Supplementary Figures S2 and S3), while the other factors of age, gender and lifestyle were not collected in the original studies used in the present meta-analysis. Second, there was a small number of original studies (especially RCTs) with not very large numbers of participants and some different follow-up periods; thus, the present study integrated the different study designs, which might undermine the meta-analysis. Third, although the measurement methods of CRP could differ across studies, many studies did not describe the methods. Thus, we did not conduct a subanalysis according to the methods, but we assumed that the studies usually did not change the methods of measuring the CRP levels before and after eradication. Fourth, the funnel plot asymmetry to detect publication bias in the literature might slightly overestimate the effect sizes. The present study findings must be carefully interpreted with these limitations, which will be addressed as the next challenge.
In conclusion, the overall meta-analysis demonstrated that H. pylori eradication was associated with reduced CRP levels; however, the result was not supported by a subgroup of the RCTs. Weak evidence exists regarding the effects of H. pylori eradication on CRP levels. Further research is warranted.