Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
OSTEOPOROSIS / BASIC RESEARCH
The Role and underlying mechanism of dental pulp stem cell-derived exosomal miR-31 in the treatment of osteoarthritis by targeting mTOR to enhance chondrocyte autophagy levels
1
Department of Orthopedics, Huashan Hospital, Fudan University, Shanghai 200040, China., China
Submission date: 2022-08-16
Final revision date: 2022-11-07
Acceptance date: 2022-11-30
Online publication date: 2023-01-14
Corresponding author
Jie Chen
Department of Orthopedics, Huashan Hospital, Fudan University, Shanghai 200040, China., China
Department of Orthopedics, Huashan Hospital, Fudan University, Shanghai 200040, China., China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
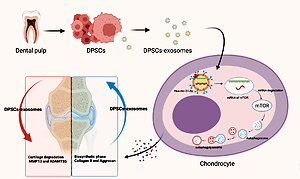
Osteoarthritis is the most prevalent musculoskeletal progressive disease that leads to functional impairment and decreased quality of life. However, the current treatments remain unsatisfactory. Recent studies reveal that exosomes derived from mesenchymal stem cells offer a promising approach to improve the pathological changes of osteoarthritis, cartilage tissue, and chondrocyte homeostasis.
Material and methods:
A randomized, controlled animal experiment was conducted on nine-week-old male C57BL/6 mice that were obtained from Charles River Laboratories. In this in vitro and in vivo study, we studied the role and mechanisms of dental pulp stem cell-derived exosomes (DPSCs-exosomes) on osteoarthritis in the mouse model.
Results:
The study findings showed that a dental pulp stem cell could generate typical-characteristic exosomes. The injection of DPSCs-exosomes ameliorated destruction of cartilage, promoted matrix synthesis, inhibited cell apoptosis, and decreased the catabolic factors expression. However, this effect was shown to be almost eliminated when miR-31 antagomir was injected.
Conclusions:
Furthermore, DPSCs-exosomes show an ability to promote autophagy in chondrocytes through mTOR inhibition, in addition to reducing the mTOR luciferase activity. The ability of DPSCs-exosomes to partially regulate autophagy was blocked upon inhibition of miR-31. In brief, DPSCs-exosomes have a chondroprotective role in the mice osteoarthritis model. The underlying mechanism is possibly related to miR-31-mediated suppression of the mTOR-autophagy pathway.
Osteoarthritis is the most prevalent musculoskeletal progressive disease that leads to functional impairment and decreased quality of life. However, the current treatments remain unsatisfactory. Recent studies reveal that exosomes derived from mesenchymal stem cells offer a promising approach to improve the pathological changes of osteoarthritis, cartilage tissue, and chondrocyte homeostasis.
Material and methods:
A randomized, controlled animal experiment was conducted on nine-week-old male C57BL/6 mice that were obtained from Charles River Laboratories. In this in vitro and in vivo study, we studied the role and mechanisms of dental pulp stem cell-derived exosomes (DPSCs-exosomes) on osteoarthritis in the mouse model.
Results:
The study findings showed that a dental pulp stem cell could generate typical-characteristic exosomes. The injection of DPSCs-exosomes ameliorated destruction of cartilage, promoted matrix synthesis, inhibited cell apoptosis, and decreased the catabolic factors expression. However, this effect was shown to be almost eliminated when miR-31 antagomir was injected.
Conclusions:
Furthermore, DPSCs-exosomes show an ability to promote autophagy in chondrocytes through mTOR inhibition, in addition to reducing the mTOR luciferase activity. The ability of DPSCs-exosomes to partially regulate autophagy was blocked upon inhibition of miR-31. In brief, DPSCs-exosomes have a chondroprotective role in the mice osteoarthritis model. The underlying mechanism is possibly related to miR-31-mediated suppression of the mTOR-autophagy pathway.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.