The latest paper concerning the management of migraine in Poland was published in 2021 [1] and the previous one (in Polish) in 2019. The Polish recommendations were based on the guidelines of migraine treatment developed by the European Headache Federation (EHF, 2019) [2] and the American Headache Society (AHS) published in 2021 [3]. The Polish guidelines took into consideration special Polish conditions and therapeutic options available in Poland, in regard to the Summary of Product Characteristics of the drugs with indication for use in migraine, the evidence-based medicine approach, good clinical practice rules, and the knowledge and experience of the experts of the Polish Headache Society (PHS/PTBG), the Headache Section of the Polish Neurological Society (HSPNS/SBGPTN), and the Polish Pain Society (PPS) [1]. It should be underlined that both the EHF and the AHF have also updated the migraine treatment guidelines/statements recently [4, 5].
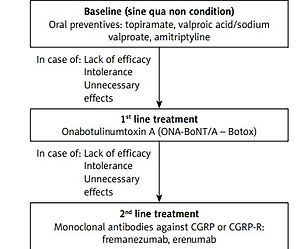
Both the above-mentioned societies have approved the use of monoclonal antibodies (mAbs) against calcitonin gene related peptide and its receptor (CGRP/CGRP-R) for the prevention of migraine in patients aged 18 years or older who are diagnosed with chronic migraine according to the criteria of the International Classification of Headache Disorders, third edition (ICHD-3) [6], or who have episodic migraine with at least 4 headache days per month [6]. Initially, in the publications from 2021 (AHS) [3] and 2019 (EHF) [2] mAbs against CGRP/CGRP-R were recommended in those who had contraindications, did not tolerate or had no response to at least 6-week treatment courses with at least two of the following medications (combining/including recommendations in episodic and chronic migraine): topiramate, valproic acid, β-blockers (metoprolol, propranolol, timolol, atenolol, nadolol), tricyclic antidepressants (amitriptyline, nortriptyline), serotonin reuptake inhibitors (venlafaxine, duloxetine) or other level A or B treatments, and, in the case of chronic migraine specifically, onabotulinum toxin type A (ONA-BoNTA), as well. In the light of the reimbursement program in Poland mentioned below [7, 8] it needs to be stressed that ONA-BoNTA is obligatory therapy used first before a patient changes the treatment to mAbs in the prophylaxis of chronic migraine. What is interesting, the latest AHS/EHF statements from this year [4, 5] clearly emphasize that the mAbs anti-CGRP/CGRP-R may be the first choice medications and there is no need for any prior treatment with the above-mentioned substances. It is important to state that many experts, including Polish national experts (PHS/PTBG, HSPNS, PPS), maintain and stated that a very good safety profile and high clinical efficacy make mAbs against CGRP/CGRP-R the first-line treatment for chronic migraine, and even for episodic pain with frequent attacks [1]. The Polish reimbursement program for the treatment of chronic migraine announced by the Ministry of Health on July 22, 2022, however, has some specific regulations. For economic reasons, this new program is based on “two lines” of treatment; however, in fact, there is a three-step therapeutic approach (Figure 1). The initial line of chronic migraine treatment includes unsuccessful treatment with at least two “classical” oral medications (out of three: valproic acid and its derivatives, topiramate, amitriptyline) and documented failure of treatment with these oral medications recommended in Polish conditions. The first line of treatment in the reimbursement program is ONA-BoNTA in the dose of up to 195 U, according to the PREEMP (PREEMPT – Phase III Research Evaluating Migraine Prophylaxis Therapy) protocol. If this therapy fails (which is evaluated after 3 cycles of therapy every 12 weeks), patients can be treated with the “second line medications” – mAbs: erenumab 140 mg s.c. monthly or fremanezumab 225/675 mg s.c. monthly/quarterly. The other two mAbs are currently not reimbursed, but galcanezumab will probably be included in this program soon, followed by eptinezumab, the only one administered intravenously every 3 months. Table I shows the drugs recommended by the PHS/HSPNS.
Table I
Drugs used for prophylactic treatment in chronic migraine, doses, and class according to PHS/HSPNS recommendation
Figure 1
Public medication reimbursement program for chronic migraine strategy for treatment of chronic migraine
Treatment efficacy should be assessed and the decision about the continuation of the treatment should be made after 3 administrations (9 months) of ONA-BoNTA or 3 months from the first administration of mAb anti-CGRP/CGRP-R. Treatment is considered to be effective if reduction in monthly headache/migraine days reaches at least 50%, relative to the pre-treatment baseline month, based on the obligatory patient’s headache diary. Functional deterioration of the patient assessed as a Migraine Disability Assessment (MIDAS) score of at least 30% is a reason to restart the therapy, after the end of treatment in accordance with the program’s regulations.
All details of the chronic migraine reimbursement program are available on websites of the Ministry of Health and the National Health Fund [7, 8], and a detailed description and interpretation with possible misinterpretation considering any doubts will be published by PHS/HSPNS in Polish, just before the program enters into force.
It is worth adding that more new medications will probably be included in the migraine recommendations soon. The small-molecule CGRP receptor antagonists known as gepants, which have shown efficacy in acute (rimegepant, ubrogepant, zavegepant) or prophylactic (atogepant, rimegepant) migraine treatment, are approved by the US Food and Drug Agency (FDA) and one of them (rimegepant) by the European Medicines Agency (EMA) for the treatment of episodic (but not chronic) migraine. Recently, rimegepant has become available in Europe and Poland in oral disintegrated tablets in the dose of 75 mg every 2 days prophylactically, or 75 mg daily as an abortive rescue.
Ditans are the next new option in abortive therapy of migraine. This novel acute migraine treatment class of medications targets the 5-HT1F receptor, but until now, the whole group has not been approved by the EMA.
Summing up, it should be emphasized that the first reimbursed migraine advanced medication treatment program is a real milestone in the attitude to migraineurs in Poland. Although it is based on economic compromise, experts in the treatment of migraine, including the chronic form of the disease, emphasize that we now have increasingly effective tools to cope with migraine, and this would significantly increase the number of people who benefit among those suffering from migraine, especially its chronic form – the most severe form of this disabling condition. Innovative drugs with higher efficacy and a superior safety profile, although also with higher costs, than standard-of-care drugs are to be promoted as they produce an indirect economic benefit by reducing loss of efficiency or work absence [9] and healthcare resource use costs. Some authors [10] observed a high discontinuation rate for migraine prophylaxis which is related to an increase in healthcare resource use and costs for non-persistent patients. Those [9, 10] recent results suggest that the standard-of-care treatment adherence implies a clinical benefit, a reduction in health resource use and costs. New therapeutic options in several years’ observation, including in real life, show high effectiveness and a better safety profile than antiepileptics and antidepressants used in CM, although it is worth emphasizing the fact of the lack of comparative studies between the options level A treatment for chronic migraine, except for topiramate [11], and the potential benefits of choosing the first drug based on other comorbidities and other factors as is considered in the American Academy of Neurology.