Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
PEDIATRICS / RESEARCH PAPER
The Efficacy and Safety of Rituximab in Children with Steroid-Dependent or Frequently Relapsing Nephrotic Syndrome: A Meta-Analysis of Randomized Controlled Trials
1
Shanghai Children's Medical Center (SCMC) affiliated to Shanghai Jiao Tong University School of Medcine, China
Submission date: 2022-11-30
Final revision date: 2023-02-09
Acceptance date: 2023-02-26
Online publication date: 2023-02-26
Corresponding author
Li Zhao
Shanghai Children's Medical Center (SCMC) affiliated to Shanghai Jiao Tong University School of Medcine, China
Shanghai Children's Medical Center (SCMC) affiliated to Shanghai Jiao Tong University School of Medcine, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
To explore the efficacy and safety of Rituximab (RTX) in children with steroid-dependent nephrotic syndrome (SDNS) or frequently relapsing nephrotic syndrome (FRNS) through meta-analysis.
Material and methods:
Meta-analysis searches were performed before November 30th, 2021, using the PubMed, Embase, Web of Science, and Cochrane Library databases to collect randomised controlled trials (RCTs). FRNS or SDNS children younger than 18 years of age were included. The RTX group was treated with RTX combined with conventional therapy, whereas the control group was given conventional therapy. Review Manager 5.3 and STATA 15.0 were used to perform the statistical analyses.
Results:
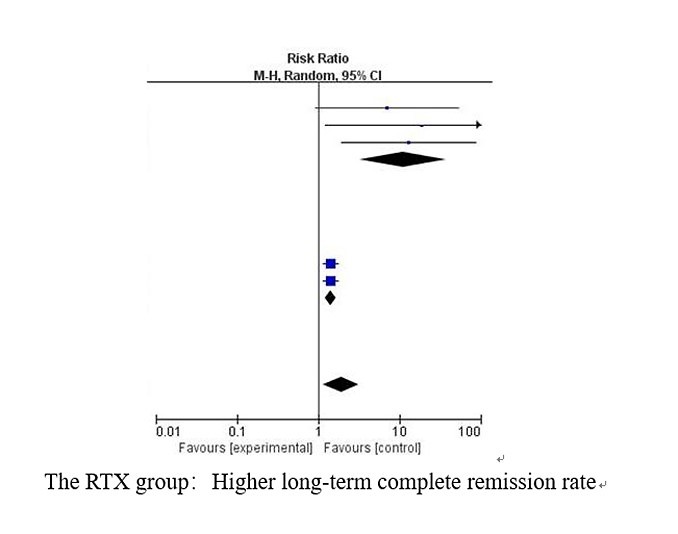
Of 1450 screened articles, a total of eight studies eligible for inclusion involving 476 patients were included. As compared to the control group, the RTX group had a significantly higher rate of long-term complete remission [RR (95% CI), 1.91 (1.16, 3.14), p<0.05]. RTX did not show significant improvement in the short term [RR (95% CI), 2.48 (0.85, 7.25), p=0.10]. However, the RTX group achieved a higher short-term complete remission rate when two studies with heterogeneity were excluded [RR (95% CI), 2.17 (1.65, 2.84), p<0.001]. Proteinuria levels were reduced more effectively in the RTX group [MD (95% CI), −1.84 (−2.42, −1.26), p <0.001)]. The RTX group and the control group had no significant differences in adverse events.
Conclusions:
RTX can be considered as an effective and safe treatment option for children with SDNS or FRNS. However, it is necessary to conduct further studies via RCTs to evaluate the persistent long-term effects.
To explore the efficacy and safety of Rituximab (RTX) in children with steroid-dependent nephrotic syndrome (SDNS) or frequently relapsing nephrotic syndrome (FRNS) through meta-analysis.
Material and methods:
Meta-analysis searches were performed before November 30th, 2021, using the PubMed, Embase, Web of Science, and Cochrane Library databases to collect randomised controlled trials (RCTs). FRNS or SDNS children younger than 18 years of age were included. The RTX group was treated with RTX combined with conventional therapy, whereas the control group was given conventional therapy. Review Manager 5.3 and STATA 15.0 were used to perform the statistical analyses.
Results:
Of 1450 screened articles, a total of eight studies eligible for inclusion involving 476 patients were included. As compared to the control group, the RTX group had a significantly higher rate of long-term complete remission [RR (95% CI), 1.91 (1.16, 3.14), p<0.05]. RTX did not show significant improvement in the short term [RR (95% CI), 2.48 (0.85, 7.25), p=0.10]. However, the RTX group achieved a higher short-term complete remission rate when two studies with heterogeneity were excluded [RR (95% CI), 2.17 (1.65, 2.84), p<0.001]. Proteinuria levels were reduced more effectively in the RTX group [MD (95% CI), −1.84 (−2.42, −1.26), p <0.001)]. The RTX group and the control group had no significant differences in adverse events.
Conclusions:
RTX can be considered as an effective and safe treatment option for children with SDNS or FRNS. However, it is necessary to conduct further studies via RCTs to evaluate the persistent long-term effects.
Share
RELATED ARTICLE