Endometrial cancer (EC) is one of the most common malignancies in women worldwide, mainly affecting women over 55 years of age. Traditionally, there are 2 types of EC: type I (endometrioid) and type II (non-endometrioid) [1]. According to literature data, about 82% of EC express hTERT, which is crucial to cell immortalisation and proliferation [2]. hTERT is a protein component of telomerase, a complex enzyme responsible for telomere lengthening in dividing cells, such as endometrial cells [3]. Its activity in postmenopausal women is rarely detected and may indicate a neoplastic process [4]. Telomeres are composed of tandem DNA repeats (TTAGGG) and proteins. They become shorter with each cell division and protect cells against chromosome fusion and genome instability, which may lead to carcinogenesis [5]. The length of telomeres differs depending on age and sex [6]. Together with other factors, TL may be a prognostic biomarker of health conditions [7]. One of the main factors that impact the dynamics of telomere shortening is chronic inflammation [8]. Shortening of telomeres has been observed in the early stage of cancer development, such as prostate cancer [9]. Several studies have demonstrated a correlation between telomere length and the risk of different cancers [10].
There are limited data regarding telomere length in endometrial cancer and hyperplasia. In published works, researchers analysed the leucocyte telomere length or compared the TL of endometrial cancer and normal endometrium. Hence, the purpose of our study was to measure the length of telomeres and the level of hTERT expression in patients with endometrial hyperplasia, endometrial cancer, and a control group, to ascertain whether the telomere measurement may indicate endometrial cancer development.
Methods
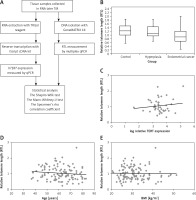
The study included 117 tissue samples from patients of the First Department of Oncology Gynaecology and Gynaecology Independent Public Clinical Hospital No. 1, Lublin, Poland. The research project received the approval of the Bioethics Committee at the Medical University of Lublin (number KE-0254/212/2020). The research included 3 groups: the control group without any lesions in the endometrium, the hyperplasia group, and the endometrial cancer group. The clinical characteristics of tissue donors are presented in Table I. Relative telomere length (RTL) was determined using the multiplex quantitative PCR, according to the procedure described by Cawthon [11, 12]. The flowchart represents the steps of analysis (Figure 1 A).
Table I
Clinical characteristics of tissue donors
Figure 1
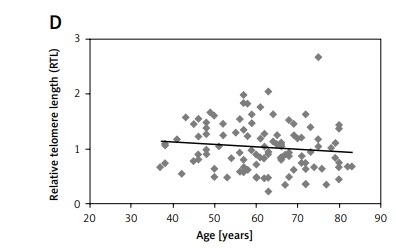
A – The flowchart of research methods. B – RTL of the control group, hyperplasia group, and endometrial cancer group. P-value = 0.78 (control vs. cancer group), 0.79 (control vs. hyperplasia group). The correlations estimated by Spearman’s Rho Calculator: C – between RTL and hTERT expression in the endometrial cancer group (the value rs is 0.780); D – between RTL and age (the value rs is –0.815)*; E – between RTL and BMI (the value rs is 0.315)*. *p-value < 0.05
Results
All analysed specimens from patients of the cancer group were identified as endometrioid adenocarcinoma. Cancer staging according to FIGO (International Federation of Gynaecology and Obstetrics) [13] was as follows: FIGO I/IA – 60.4%, FIGOIB – 22.6%, and FIGO II–-IV – 16.6%. Tumour differentiation grades were as follows: G1 – 44.1%, G3 – 50%, G3 – 5.9%. The distribution of variables was analysed by the Shapiro-Wilk test, which indicated that the data were not normally distributed (W = 0.74, p < 0.01).
The comparison of RTL indicated that the longest telomeres were in the control group – 1.22 (1.00–1.48). Slightly shorter telomeres were observed in the hyperplasia group – 1.09 (0.95–1.44). The shortest telomeres were seen in the endometrial cancer group – 0.89 (0.67–1.21), which also showed strong variability of telomere length (Figure 1 B). Although a trend towards telomere shortening in the hyperplasia and cancer groups was visible, the differences were not statistically significant.
The RTL in the groups depending on staging and grading according to FIGO was as follows: in the FIGO I–IA group – 0.95 (0.72–1.29); in the FIGO IB group – 0.72 (0.49–0.93); and in the FIGO II-IV group – 0.88 (0.69–1.19). The median RTL in different grading groups was as follows: in the G1 group – 0.91 (0.62–1.22); in the G2 group – 0.89 (0.69–1.24); in the G3 group – 0.75 (0.55–0.98). The correlation between RTL and other parameters was analysed using the Spearman’s Rho (Correlation) Calculator. A negative correlation was observed between the RTL and patient’s age (rs = –0.815) (Figure 1 D), while a weak positive correlation was observed between RTL and BMI (rs = 0.315) (Figure 1 E).
The expression of hTERT was assayed using the Relative Standard Curve method with GAPDH as the reference gene. There was no hTERT expression in the control and endometrium hyperplasia groups. In the endometrium cancer group, 44% of samples expressed hTERT. Patients with EC and hTERT expression were older than patients with EC and without hTERT expression: 67 years (60–74.25) vs. 63 years (57–71). In the first group the median number of years after menopause was 13 (8–26), while in the subgroup without hTERT expression it was 10 (6–11.5). About 97% of the subgroup with hTERT expression had a BMI over 25 (in comparison to subgroup without hTERT expression). There was a weak positive correlation between relative telomere length (RTL) and telomerase expression in the endometrial cancer group (Figure 1 C).
Discussion
In the current study, the shortest and the most varied telomeres were in the cancer group, but the differences in RTL between groups were not statistically significant. This observation is consistent with the previous study conducted by Maida et al. [14], who suggested that telomere length is stable in endometrial hyperplasia and cancer. Moreover, the length of telomeres was correlated with hTERT expression, which occurred in 44% of cancer specimens. It confirms previous indications that telomerase reactivation is common in endometrial cancer [15]. The lack of hTERT expression in hyperplasia suggests that telomerase reactivation may occur in a later stage. The observed tendency of telomere shortening in cancer cells may reflect previous research that included cervical cancer, in which the shortest telomeres were found in cancer cells in the first stage of development [16].
We did not observe significant differences in telomere length depending on EC cancer stage or tumour differentiation grade. This may be because, according to FIGO, analysed specimens represented mostly stage I (I–IB). Therefore, we did not identify analysis of telomere length as an indicator of endometrioid cancer progression. Our results are convergent with outcomes published by Benati et al. [17], who analysed RTL of cell-free DNA (cfDNA) for endometrioid EC and did not observe significant differences in RTL among EC stages or grades.
The strength of our research is the homogenous cancer group – all specimens were endometrioid type cancer. Almost 64% of patients in our EC cohort were overweight (BMI > 25 kg/m2) and 25% of patients were obese (BMI > 35 kg/m2), and interestingly we observed a relationship between telomere length and patients’ BMI. This observation may reflect preceding results showing that obesity may increase oestrogen levels in postmenopausal women, as well as the risk of EC [3, 18]. EC patients with hTERT expression were older and more overweight than patients without hTERT expression. This is also the first report in which telomere length was analysed in normal, hyperplasia, and cancer tissues by the qPCR method in a single study. Previous studies presented data of normal and cancer specimens or the length of leucocyte telomeres of cancer and normal patients.
In conclusion, the present study indicates that the analysis of telomere length may not be an indicator of endometrioid cancer development. Further research is needed to shed more light on the role of telomere in endometrial cancer development.