Introduction
Skin cancers are one of the most prevalent malignancies in the world, with a high incidence in the United States, Australia, and Europe [1–3]. The incidence rates of skin cancers vary by the type of tissue in which they arise, with the incidence rates of melanoma, basal cell carcinoma, and squamous cell carcinoma being the highest [4]. Moreover, rapid industrialization worldwide has led to the destruction of part of the ozone layer, which is thought to contribute to the year-on-year increases in the incidence rates of skin cancers, making them a global public health problem [5].
The systemic immune-inflammation index (SII) is a newly developed indicator that is calculated based on counts of peripheral blood neutrophils, lymphocytes, and platelets, and more accurately reflects the inflammatory status and immunological balance of the body than other indicators [6]. Consequently, the SII is useful for evaluating the prognosis of certain cancers, such as liver, lung, gastric, esophageal, urinary system, and cervical cancers [7–11], and its clinical utility has become increasingly recognized. Moreover, SII tests are non-invasive, inexpensive, and can help to identify patients at high risk of cancer. However, there are few reports on the relationship between SII values and skin cancers. In one study, we found differences in various inflammatory markers, including SII, in melanoma patients [12].
We speculated that high SII values would be associated with high susceptibility to skin cancers. Therefore, in this study, we evaluated the dose–response relationship between SII values and the occurrence of skin cancers in U.S. adults by analyzing National Health and Nutrition Examination Survey (NHANES) data for 2010–2018. We also determined the impact of underlying moderators on this association.
Material and methods
Study population
The study population comprised participants in NHANES, which is an ongoing survey employing a sophisticated, multi-stage probability sampling technique to evaluate the nutritional and physical health of a representative sample of the non-institutionalized general population of the USA. The NHANES survey protocol has been approved by the National Center for Health Statistics Ethics Review Board, and each participant signs a written informed consent form. The NHANES data include information on demography, diet, physical examinations, and laboratory tests. The current study integrated NHANES data for 2010–2018, using the NHANES analysis guidelines for cross-sectional research.
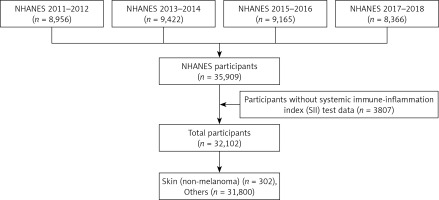
Figure 1 depicts the procedures that were applied to select the participants for the current study. Potential participants who were aged younger than 20 years, pregnant, or lacked SII data were excluded. The study sample comprised 32,102 participants who were aged 20 years or older.
Covariates
Demographic data were collected on age, sex, ethnicity, poverty-to-income ratio, education level, and marital status. Self-reported information on ethnicity was used to divide the participants into groups comprising Mexican-American, non-Hispanic White, non-Hispanic Black, and other ethnicities. Education levels were classified based on self-reported information as below high-school level, high-school level, or above high-school level. Marital status was classified based on self-reported information as married/cohabiting, widowed/divorced/separated, or unmarried. Smoking (never/past/current) and alcohol consumption information (never/past/current) was extracted from questionnaire answers. Body mass index (BMI) was computed by dividing body weight in kilograms by the square of height in meters. According to criteria suitable for the U.S. population, BMI values below 18.5 kg/m2, above 25 kg/m2, 25–29.9 kg/m2, and over 30.0 kg/m2 were categorized as underweight, normal, overweight, and obese, respectively.
The parameters comprising the SII, namely lymphocyte, neutrophil, and platelet counts, were measured by performing comprehensive blood counts of samples with a concentration of 1,000 cells/µl using an automated hematologic analyzer (DxH-800 Hematology Analyzer, Beckman Coulter, Brea, CA, U.S.A.). The detailed methodologies of this and other laboratory tests are provided on the NHANES website [13]. The SII was calculated using the following equation [14]: SII = (platelet count × neutrophil count)/lymphocyte count.
Outcomes
This cross-sectional study utilized data selected from the NHANES 2010–2018, as the NHANES follows a 2-year cycle. A diagnosis of skin cancer in the NHANES is determined by asking the following questions: “{Have you/Has the standardized patient} ever been informed by a doctor or other health professional that {you/she/he} had skin cancer?” and “How old were you when you were first informed that you had skin cancer?”
Statistical analysis
According to the NHANES guidelines, when merging data from two or more cycles, new sample weights need to be recalculated. In this study, data from the NHANES 2010–2018 were merged, and descriptive variables are presented as number of patients with percentages or median and interquartile range. Therefore, the data were analyzed using complex weights. Non-normally distributed continuous variables are presented as medians with quartile intervals and were compared using Wilcoxon rank sum tests. Categorical variables are presented as frequencies and were compared using χ2 tests.
A logistic regression model was used to analyze the relationship between SII values and skin cancers. A one-unit change in the logistic regression dependent variable causes the amount of change in Y or the association to be often small and not easily captured. To increase the model’s ability to detect an association, we used quartile spacing (Q), where we grouped the independent variables X in quartile spacing, with the first quartile group as a benchmark [13, 14].The outcomes are reported as odds ratios (ORs), 95% confidence intervals (CIs), and p-values. Model 1 was not adjusted for any possible confounding factors. Model 2 was adjusted for sex, ethnicity, and annual household income. Model 3 was adjusted for sex; ethnicity; annual household income; and alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total cholesterol, triglyceride, immunoglobulin G (IgG), glucose, creatinine, and total calcium concentrations.
To account for underlying moderators, a subgroup analysis was conducted based on age (< 60 or ≥ 60 years), sex (female or male), ethnicity (non-Hispanic White or other ethnicity), level of education (above high-school, high-school, or less than high-school level), marital status (never married, married, divorced, or other), and annual household income (< US$20,000, US$20,000–44,999, US$45,000–74,999, or US$75,000–100,000).
A bilateral p-value of less than 0.05 was regarded as indicating statistical significance. All of the statistical analyses were conducted using R software (version: 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant screening and baseline characteristics
The baseline data of participants are listed in Table I. The average age was 33.00 (13.00, 56.00) years; 49.32% of participants were men and 50.68% were women. There were significant differences (p < 0.05) between participants with melanoma and those with non-melanoma skin cancers in terms of age; sex; race; education level; marital status; annual family income; creatinine, glucose, globulin, triglyceride, total cholesterol, AST, and ALP concentrations; lymphocyte, neutrophil, and platelet counts; and SII value.
Table I
Baseline characteristics of participants by skin cancer (non-melanoma), NHANES 2010–2018 (n = 32102)
The single-factor logistic regression model analysis results for each factor are listed in Supplementary Table I. It can be seen that association factors for non-melanoma skin cancers were age over 60 years (OR = 11.26, 95% CI = 8.71–14.57, p < 0.001); non-Hispanic White ethnicity (OR = 19.73, 95% CI = 9.31–41.80, p < 0.001); a high-school or general level of education (OR = 5.53, 95% CI = 3.06–9.96, p < 0.001) or an above-high-school level of education (OR = 12.05, 95% CI = 6.88–21.10, p < 0.001); an annual family income of US$45,000–74,999 (OR = 1.90, 95% CI = 1.29–2.82, p = 0.001) or more than US$100,000 (OR = 2.21, 95% CI = 1.51–3.25, p < 0.001); a low concentration of triglycerides (OR = 1.08, 95% CI = 1.02–1.14, p = 0.006), a high concentration of total cholesterol (OR = 1.17, 95% CI = 1.06–1.29, p = 0.001), or a low concentration of high-density lipoprotein cholesterol (OR = 1.44, 95% CI = 1.10–1.87, p = 0.007); and an SII value in the third quartile (OR = 1.62, 95% CI = 1.13–2.34, p = 0.009) or fourth quartile (OR = 2.51, 95% CI = 1.79–3.52, p < 0.001). In contrast, protective factors against non-melanoma skin cancer were female sex (OR = 0.80, 95% CI = 0.63–1.00, p = 0.049); non-Hispanic Black ethnicity (OR = 0.20, 95% CI = 0.04–0.97, p = 0.045); a marital status of unmarried (OR = 0.21, 95% CI = 0.13–0.33, p < 0.001); and a low concentration of globulin (OR = 0.90, 95% CI = 0.87–0.92, p < 0.001) (p < 0.05 for interaction).
Comparison of SII values with various factors
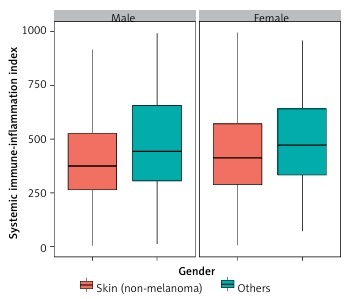
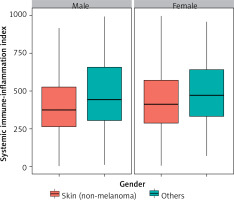
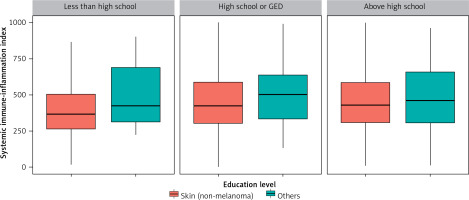
Figure 2 shows the distribution of SII values according to skin cancers and sex. Men with melanoma had higher SII values compared to men with non-melanoma skin cancers. The same association was observed for women, although the statistical dispersion was smaller than in men. Figure 3 illustrates the distribution of SII values for skin cancers based on education level. Among individuals with a below-high-school education, the statistical dispersion of SII values for those with melanoma was greater than for those with non-melanoma skin cancers. However, the median SII values were similar for these groups. For participants with a high-school or general education level, the statistical dispersion of SII values was similar to that for those with non-melanoma skin cancers. However, the median and average values of the former were slightly higher than those of the latter. Among participants with an above-high-school education level, the statistical dispersion of SII values for melanoma was slightly larger than that of those with non-melanoma skin cancers, but their medians were similar.
Association between SII values and non-melanoma skin cancers
A logistic regression method was employed to analyze the association between SII values and non-melanoma skin cancers. Table II displays this association, as determined by three logistic regression models. In Model 1, the association of the first and second quartiles of SII values and the occurrence of non-melanoma skin cancers was higher than that between the third and fourth quartiles of SII values and the occurrence of non-melanoma skin cancers (OR = 2.507, 1.785–3.522, p < 0.001). In Model 2, the same trend was present. In Model 3, the positive correlation between the highest quartile of SII values and the occurrence of non-melanoma skin cancers was slightly lower than in the other models (OR = 1.650, 1.158–2.352, p = 0.006).
Table II
Logistic regression analysis of the relationship between skin cancer (non-melanoma) and SII
[i] Model 1: did not adjust for any confounding factors. Model 2: adjusted for sex, race, annual household income. Model 3: adjusted for sex, race, annual household income, ALT, AST, ALP, total cholesterol, triglyceride, globulin, glucose, creatinine, total calcium. Systemic immune-inflammatory index quartile ranges: quartile 1: 1.53–282.00; quartile 2: 282.01–406.36; quartile 3: 406.37–584.57; quartile 4: 585.58–9540.00.
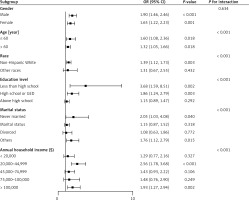
Figure 4 presents the results of our subgroup analysis and interaction test, which investigated whether demographic characteristics could explain the association between the natural logs of SII values and ORs of non-melanoma skin cancers. There were statistically significant associations (all p < 0.05) between subgroups stratified by age and the occurrence of melanoma. Regarding the subgroups stratified by ethnicity, education level, marital status, and annual family income, there was a statistically significant and positive relationship between subgroups comprising those of non-Hispanic White ethnicity, with a below-high-school or general level of education, with an unmarried or another marital status, or with an annual family income of US$20,000–44,999 or more than $100,000, respectively, and the occurrence of melanoma. There was also a statistically non-significant (p > 0.05) positive relationship between SII values and the occurrence of melanoma in the remaining subgroups.
The results of the cross test revealed that neither sex nor hyperlipidemia affected the positive relationship between high SII values and the association of melanoma (p > 0.05 for the cross test). Conversely, the positive relationship between high SII values and the occurrence of non-melanoma skin cancer was increased by an age of 60 or younger (OR = 1.60, 1.08–2.36, p = 0.018) or over 60 (OR = 1.32, 1.05–1.66, p = 0.018), non-Hispanic white ethnicity (OR = 1.39, 1.12–1.73, p = 0.003), a below-high-school (OR = 3.68, 1.59–8.51, p = 0.002) or high-school or General Education Development level of education (OR = 1.86, 1.24–2.79, p = 0.003), a marital status of never married (OR = 2.05, 1.03–4.08, p = 0.040) or other (OR = 1.76, 1.12–2.79, p = 0.015), and an annual household income of US$20,000–44,999 (OR = 2.56, 1.78–3.68, p < 0.001) or over US$100,000 (OR = 1.93, 1.27–2.94, p = 0.002) (p < 0.05 for interaction) (Supplementary Table SI).
Discussion
This cross-sectional study analyzed 2010–2018 data on 32102 NHANES participants to explore the association between SII values and the occurrence of skin cancers. The main findings were as follows. (1) Age, ethnicity, and education level were the factors most associated with the association of non-melanoma skin cancers. (2) In both men and women, the SII values of those with melanoma were generally higher than the SII values of those with non-melanoma skin cancers, and this association was also present in those who had a below-high-school level of education. (3) After adjustment for confounding factors, high SII values were positively associated with the occurrence of non-melanoma skin cancers, although this association was slightly lower than that before adjustment. (4) Subgroup analyses and interaction tests revealed that a high SII value was associated with a high occurrence of non-melanoma skin cancers, and this association was affected by age, race, education level, marital status, and annual family income.
Studies have indicated that tumorigenesis and tumor development are caused by chronic inflammation, which is an abnormally prolonged protective response to tissue imbalance. The process of tumorigenesis and tumor development involves genotoxicity; abnormal tissue repair; proliferative responses; and tumor-mediated immunosuppression, invasion, and metastasis [5, 15–17]. Recently, the tumor microenvironment has received increasing attention in the field of tumor immunology, as its significant elements include a variety of inflammatory cells and mediators [15]. The SII is calculated based on the counts of peripheral neutrophils, platelets, and lymphocytes; thus, compared with other measures, it is a more comprehensive and objective representation of the balance between the immune system activity and the level of inflammation in the body. High SII values are correlated with high platelet or neutrophil counts and/or low lymphocyte counts. Thus, a high SII value indicates the possible presence of a tumor microenvironment with a high level of inflammation and invasive immune cells, and thus a decreased immune response in the body [17, 18]. In addition, immune cells that infiltrate into a tumor microenvironment with a high level of inflammation promote tumor growth [19, 20]. Many studies have examined the association between SII values and tumors, but few studies have examined the association between SII values and non-melanoma skin cancers. Therefore, investigation of the latter association was the main focus of the current study, given that skin cancers comprise malignant melanomas and non-melanoma skin cancers [21].
Non-melanoma skin cancers consist of a large number of malignant tumors, such as endometrial, blood, blood vessel, neuroendocrine, accessory gland, and epithelial cancers. The skin is also a major site of internal tumor metastasis [22]. It has been found that the age shift in the population has resulted in an overall increase in total number of skin cancers, since the incidence of non-melanoma skin cancer increases with age. Indeed, 80% of cases occur in people aged 60 years and older. Those over 60 years of age may have a greater susceptibility to non-melanoma skin cancers [23–26]. This is similar to the results of the present study, where increasing age affects the positive association between high SII values and non-melanoma skin cancers. Moreover, chronic inflammation, known as “inflammation”, may be a major cause of cancer due to the association with aging processes. Specifically, inflammatory processes can induce oxidative stress and reduce the antioxidant capacity of cells. Excess production of free radicals can damage fatty acids and proteins in cell membranes, permanently impairing cellular function. Free radicals can also damage DNA and thereby cause mutations, which may lead to cancer and age-related diseases. Moreover, SII values increase by 15 as people age, and this may also increase the risk of cancer [27]. Furthermore, aging is often accompanied by circadian rhythm disorders, which are associated with a decrease in the ability to maintain cellular pressure at both local and global levels, which increases the risk of tumor growth [28].
Skin cancers are less common in other ethnicities than in white people with light skin [5, 22, 29], as the former have higher epidermal concentrations of melanin, which protects the skin from damage due to sunlight exposure. Thus, the inherent sun-protection factor of Black people is as high as 13.4 [30]. Melanin also has antioxidant- and free radical-scavenging activities, which may reduce the impact of inflammation. Therefore, the lower the concentration of melanin in the skin, the higher is the risk of developing skin cancers.
Our findings showed that the participants with a level of education above high school were more likely to suffer from melanoma than those with other levels of education. Ultraviolet radiation is a key cause of skin cancers [31]. However, our findings showed that compared with participants with an education level of high school or below, those with an education level of high school or above protected themselves 22% less against sunlight via wearing sun screening clothing and staying in the shade. This may increase the latter group’s risk of non-melanoma skin cancers. Research has shown that public awareness of non-melanoma skin cancers and their prevention are limited, and health information on the Internet is not always tailored to the needs of different ethnic groups in terms of its quantity and quality [32]. Therefore, people with a high education level may be insufficiently aware of the risk of, or have inadequate access to information on, non-melanoma skin cancers, such that they do not adopt the necessary self-protective behaviors.
Studies have used a variety of epidemiological methods and target populations to demonstrate the association between high SII values and the association of cancers, such as non-small-cell lung cancer, pancreatic cancer, and melanoma [12, 17, 33]. Similarly, our findings showed that high SII values were positively associated with non-melanoma skin cancers in the selected NHANES participants. Furthermore, subgroup analyses and interaction tests clarified that this association was affected by age, ethnicity, education level, marital status, and annual family income.
Most people receive approximately one fifth of their total lifetime sunlight exposure during childhood. Thus, the total sunlight to which people are exposed over time and the adoption of sunbathing may lead to increases in SII values – and thus due to immunosuppression and photoaging of the skin, the risk of non-melanoma skin cancers is associated with people’s age.
Education level is a fundamental social determinant of a person’s employment, salary, societal relationships, knowledge about health, and cognition skills. Compared with educated people, uneducated people lack social resources and therefore are more prone to developing unhealthy behaviors; they also lack healthcare resources. Furthermore, low income and education levels are associated with reduced use of clinical prevention services and increased lifestyle-related health associations. This means that compared with well-educated high-income people, less-educated low-income people may be more likely to have unhealthy behaviors and under-use health services, and thus be more likely to exhibit an association between high SII values and the occurrence of non-melanoma skin cancers. Studies have revealed that compared with other subpopulations, non-Hispanic white and unmarried respondents are more likely to report a history of sunburn [34]. This may account for the positive association between high SII values and occurrence of non-melanoma skin cancers in these subpopulations.
Due to the fact that the data involved in SII is easily obtained from routine blood testing, and routine blood measurement has low cost, good feasibility, and repeatability, SII is considered to objectively reflect the balance between host inflammation and immune status. This study confirms a positive correlation between high SII and skin cancer, but more research is needed to determine its specific value in clinical practice and public health. In addition, further research is needed on its interaction with other biomarkers or treatment methods. In summary, SLL has potential value in the clinical and public health aspects of skin cancer, but more research is needed to determine its exact role and application. In future research, we can further focus on the issue of skin cancer in different regions and populations, as well as how to develop more effective public health strategies to address this challenge. Firstly, we need to further explore the pathogenic mechanisms of skin cancer, particularly the interactions between genetic, environmental, and biochemical factors. Secondly, we need to strengthen monitoring and management of high-risk groups to improve early diagnosis and treatment effectiveness. In addition, we will continue to promote technological innovation, such as using artificial intelligence for skin cancer detection, and developing new treatment methods and drugs. Finally, we need to strengthen public education and publicity to raise people’s awareness of skin cancer and its prevention.
Several potential mechanisms may be used to explain the prognostic values of SII in tumor. Studies have shown that myelofibrosis, malignancy, anemia, trauma, and surgery can lead to elevated white blood cell counts in routine peripheral blood markers. Platelets are one of the formed components of blood, and when inflammation exists in the body, it can induce activation of the coagulation pathway, and thrombin can bind to the platelet surface protease activation receptor to initiate platelet activation, aggregation, and the release of pro-inflammatory and chemotactic factors to participate in the inflammatory response [35]. When the dysregulation of the host response to infection produces a large number of inflammatory factors, tumor necrosis factor α, interleukin 1β, interleukin 6 and various other inflammatory factors are released in large quantities, triggering a systemic inflammatory response and a sharp increase in the number of neutrophils, and at the same time, inflammatory cytokines can induce immunosuppression, which subsequently leads to apoptosis of a large number of lymphocytes, and thus the balance between neutrophils and lymphocytes is disrupted. Based on the above theory, SII should be a more objective marker that reflects the balance between host inflammatory and immune response status than all the other systemic inflammation indices such as the platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR).
This study had the following strengths. First, it had a large sample size comprising a typical range of the U.S. population. Second, confounding factors were taken into account, thereby enhancing the reliability of our findings. However, this study had the following limitations that necessitate careful interpretation of our findings. First, causality between SII and non-melanoma skin cancers could not be established because of the cross-sectional design of this study. Thus, a more in-depth study is needed to explore such relationships. Second, some information on covariates was collected based on self-reported questionnaires, which might not accurately reflect the actual situation and may introduce recall bias. Third, although we controlled for several confounding factors, some confounding variables (such as C-reactive protein and sex hormone levels) were not included finally because they were not available in the NHANES database. Last, the data of the cross-sectional study were collected at a certain point or in a short period of time, but the data collection period in this paper was 2010–2018; therefore, the results should be interpreted with caution.
In conclusion, our findings show that certain ages, races, levels of education, marital status, and annual household income levels affect the positive association between high SII values and non-melanoma skin cancers. Therefore, effective measures should be taken to protect susceptible populations from non-melanoma skin cancers.