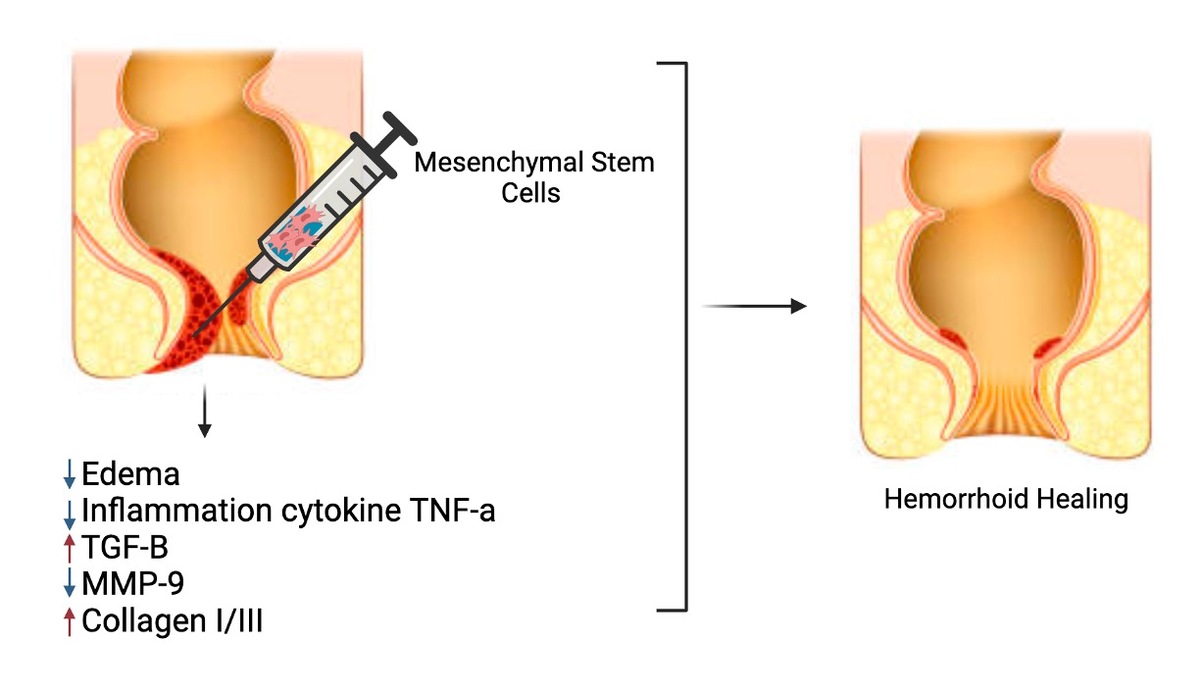
Introduction
Hemorrhoids, one of the oldest and most common anorectal diseases, affect a significant portion of the global population [1]. An estimated 10 million people suffer from hemorrhoids, with a prevalence of about 4.4%, making it the third most frequent diagnosis in emergency departments and outpatient visits worldwide [2, 3]. In Indonesia, the estimated prevalence of hemorrhoids is 5.7%, affecting around 12.5 million people, yet only 1.5% receive a diagnosis due to various reasons, including being asymptomatic or feeling embarrassed to seek treatment [4]. The etiology of hemorrhoids has been the subject of numerous studies, leading to a lack of a targeted therapeutic approach [5]. Hemorrhoids are vascular cushions in the submucosal layer of the anal canal. Hemorrhoids become symptomatic when they swell, become inflamed, or cause pain, especially when they prolapse into the anal canal [1, 6].
Treatment options for hemorrhoids, particularly those that have prolapsed, mainly involve surgical therapy, such as hemorrhoidectomy [7]. The administration of phlebotonics (flavonoids), a micronized purified flavonoid fraction (MPFF) such as a combination of diosmin and hesperidin, is a non-operative therapeutic option widely used to reduce bleeding, swelling, and pain in hemorrhoids [8–11]. However, the effectiveness of these flavonoids in improving collagen deposition in hemorrhoids has not been proven. Histopathological examination of hemorrhoidal specimens reveals venous dilatation, thrombosis, and damage to the supporting connective tissue and mucosal suspensory ligament of Treitz [7, 12]. Damage to the supporting connective tissue of hemorrhoids can result in inadequate fixation of anal cushions, leading to their prolapse into the anal canal [13]. Prolapsed anal cushions, when entrapped by the anal sphincter muscle, can undergo strangulation, resulting in stasis or impaired blood flow to the anal cushions [1, 14]. This condition can trigger inflammation in the vascular wall and anal cushions surrounding tissue, marked by the release of reactive oxygen species, release of inflammatory mediators, activation of acute inflammatory cells, and MMP-9 enzyme, which can degrade the extracellular matrix and lead to ulceration [15, 16].
Mesenchymal stem cells (MSCs) have been extensively researched for their ability to suppress inflammation, expedite proliferation, and shorten the remodeling phase in wound healing [14–22]. Previous studies reported that the MSCs derived from adipose tissue or bone marrow have shown promising results in the treatment of perianal fistulas, and the secretome of MSCs has been found to restore tissue expression in hemorrhoid rats [23]. Additionally, MSCs have been associated with improved healing in perianal Crohn’s disease [24]. These findings suggest that MSCs hold potential for the treatment of hemorrhoids and other anorectal conditions. Therefore, this study aims to evaluate the capacity of mesenchymal stem cells in repairing hemorrhoids in a rat animal model compared to the administration of diosmin-hesperidin therapy through macroscopic assessment, histopathology, and molecular analysis.
Material and methods
MSC culture and isolation
Rat MSCs were obtained from a female rat on the 19th day of pregnancy. In brief, donor rats underwent anesthesia, and their abdomens were dissected. Under sterile conditions, the umbilical cord (UC) was gathered and rinsed in phosphate-buffered saline (PBS) (Gibco Invitrogen, NY, USA). Subsequently, the UC artery and vein were excised, and the UC was then cut into 2–5 mm lengths using a sterile scalpel. These sections were evenly distributed in T25 flasks containing Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, St. Louis, MO) supplemented with 10% PBS, 100 IU/ml penicillin/streptomycin (Gibco Invitrogen, NY, USA), and incubated at 37°C with 5% CO2. The medium was refreshed every 3 days, and cells were passaged when reaching 80% confluence. For subsequent experiments, UC-MSCs at passages 4–6 were utilized [17, 20]. The MSCs still retained their regenerative potential, immunomodulatory properties, safety, and efficacy in optimal conditions on passages 4–6 of culture. Additionally, the MSCs had not yet undergone differentiation to a mature state.
Osteogenic and adipogenic differentiation assay of MSCs
The MSCs were cultured in a 24-well plate at a density of 1.5 × 104 cells per well using a standard medium composed of DMEM (Sigma-Aldrich, St. Louis, MO), supplemented with 10% FBS (Gibco Invitrogen, NY, USA), and 1% penicillin (100 U/ml)/streptomycin (100 µg/ml) (Gibco Invitrogen, NY, USA). The culture was maintained at 37°C, 5% CO2, and ≥ 95% humidity. Upon reaching 80% confluence, osteogenic and adipogenic differentiation protocols were initiated. For osteogenic differentiation, the standard medium was replaced with an osteogenic differentiation medium consisting of Human MesenCult Osteogenic Differentiation Basal Medium (Stem Cell Technologies, Singapore), supplemented with 20% Human MesenCult Osteogenic Differentiation 5X Supplement (Stem Cell Technologies, Singapore), and 1% L-glutamine (Gibco Invitrogen, NY, USA). The differentiation medium was refreshed every 3 days. Visualization of osteogenic differentiation was achieved by staining with 1 ml of 2% alizarin red solution after bone matrix formation. For adipogenic differentiation, the growth medium was switched to Human MesenCult Adipogenic Differentiation Basal Medium (Stem Cell Technologies, Singapore). The medium was changed every other day, and on day 35, cultures were stained with Oil Red O and examined under a microscope [21, 25, 26].
Characterization of MSC surface markers
MSCs underwent analysis for the expression of specific surface markers using flow cytometry. In brief, the cultured cells were incubated in the dark with primary antibodies, including mouse anti-human CD29 (BD Biosciences, San Jose, CA, USA), mouse anti-human CD90 (BD Biosciences, San Jose, CA, USA), and mouse anti-human Lin negative (CD45/CD31) (BD Biosciences, San Jose, CA, USA), followed by secondary conjugated antibodies. After staining with specific antibodies for 30 min at 4°C, the cells were examined using a BD Accuri C6 Plus flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using BD Accuri C6 Plus software (BD Biosciences, San Jose, CA, USA) [27–29].
Croton oil-induced hemorrhoid animal model
The experiments were conducted using a modified methodology derived from the description by Azeemuddin et al. (2014) to induce hemorrhoids in mice employing croton oil. The mice were randomly divided into six groups, each comprising five animals. The first group, denoted as the healthy group (Healthy), consisted of healthy mice. Two negative control groups were established: C-1, in which hemorrhoids were induced by gently rubbing a sterile cotton swab in the anorectal area for 30 s once a day for three consecutive days and subsequently treated with vehicle CMC-Na orally for five consecutive days (C-1 group for controlling groups C+ and T2 administered orally); and C-2, croton oil-induced hemorrhoids were treated with single dose perianal injection of vehicle NaCl 0.9% (C-2 group for controlling groups T1 and T2 administered by perianal injection). The positive control group (C+) comprised croton oil-induced hemorrhoid mice treated with diosmin-hesperidin orally for five consecutive days. Two treatment groups were included: T1, mice with croton oil-induced hemorrhoids treated with a single dose perianal injection of 1.5 × 106 MSCs on day 4; and T2, mice with croton oil-induced hemorrhoids that received a combined treatment of diosmin-hesperidin along with MSCs. The induction of hemorrhoids using croton oil involved a mixture containing 200 µl of croton oil (Sigma-Aldrich, St. Louis, MO), deionized water, pyridine (Sigma-Aldrich, St. Louis, MO), diethyl ether (Sigma-Aldrich, St. Louis, MO), and 6% croton oil in a ratio of 1 : 4 : 5 : 10. On day 9, all mice were euthanized, and rectoanal resected tissue were isolated for further analysis [23, 30].
Protein expression analysis by western blot
The rectoanal tissues that were isolated underwent lysis in RIPA buffer (Thermo Fisher Scientific, USA), and the protein concentration was determined using the Pierce BSA CBB Assay. Portions containing 10 µg of total protein were combined with 2× Laemmli buffer (Bio-Rad Laboratories, California, USA) at a 1 : 1 ratio, boiled, and then separated on 10% SDS-PAGE gels (Bio-Rad Laboratories, California, USA). The separated proteins were transferred to polyvinylidene fluoride (PVDF) membranes, which were subsequently blocked with 5% bovine serum albumin (BSA) (Sigma Aldrich, St. Louis, MO) in phosphate buffered saline with Tween (PBST) (Sigma Aldrich, St. Louis, MO) for 1 h. TNF-α antibodies (Santa Cruz Biotechnology, Texas, USA), TGF-β antibodies (Santa Cruz Biotechnology, Texas, USA), collagen type I antibodies (Santa Cruz Biotechnology, Texas, USA), and collagen type III antibodies (Santa Cruz Biotechnology, Texas, USA) were individually applied to the blocker overnight at dilutions of 1 : 1000. Subsequently, the membranes were washed, incubated with HRP-conjugated secondary antibody (GeneTex Biotechnology, USA), washed again, incubated with ECL (Bio-Rad Laboratories, California, USA) reagent, and exposed to chemiluminescence. The chemiluminescence signal was captured using the Invitrogen IBright ChemiDoc Imaging System [31–33].
Gelatin zymography assay
Gelatin zymography was conducted to assess the activity of matrix metalloproteinases (MMPs) in the isolated rectoanal tissue. The tissues were harvested, and protein extraction was performed using RIPA buffer. The proteins were separated on an 8% SDS-PAGE gel under non-reducing conditions, and the gel was supplemented with 0.1% gelatin (Sigma-Aldrich, St. Louis, MO). In each gel run, an equivalent amount of protein (150 µg) was utilized. Following electrophoresis at room temperature, the gel was immersed for 30 min in a 2% Triton-X 100 solution (Merck Millipore, USA) before being incubated in 100 ml of incubation buffer containing 40 mM Tris HCl, pH 8, 10 mM CaCl2, 0.02% NaN3 for 24 h at 37°C. Subsequently, the gel was stained with Coomassie Brilliant Blue R-250 solution for 2 h and then destained (20% methanol, 10% acetic acid, and 70% water) until bands with a clear appearance on a dark blue background indicated the gelatinolytic activity of MMP-9. The band intensity was quantified using ImageJ (Molecular Graphics Laboratory, The Scripps Research Institute) [34].
Statistical analysis
The data were expressed as the mean ± SD. Statistical analyses to assess differences between groups were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA) with ANOVA and post-hoc Fisher’s least significant difference analysis. A significance level of p < 0.05 was considered statistically significant. Graphical representations were generated using GraphPad Prism version 10 (Boston, USA).
Results
MSC isolation and characterization
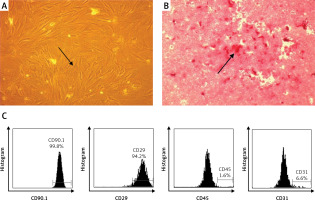
MSCs were analyzed based on their plastic adherent capability under standard culture. MSCs (passage 4) showed adherent cells with typical monolayers of spindle-shaped fibroblast-like cells (Figure 1 A). To confirm the in vitro differentiation potential of MSCs, we used osteogenic differentiation media to evaluate whether MSCs can differentiate into osteogenic cells. We observed a red color in the osteogenic differentiation assay as calcium deposition, indicating that the MSCs differentiate into osteogenic cells (Figure 1 B). Immunophenotyping MSCs using flow cytometric analysis indicated that MSCs were positive for CD90 (99.80%) and CD29 (94.20%), and negative for CD45 (1.65%) and CD31 (6.60%) (Figure 1 C).
The combination of MSCs and diosmin-hesperidin treatment inhibited tissue edema
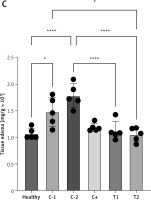
The measurement of tissue edema (rectoanal coefficient) was conducted by comparing the resected rectoanal tissue weight (up to 20 mm length from the anal opening) to the body weight of the experimental mice. The mean tissue edema increased in the negative control/hemorrhoid groups treated with vehicle CMC-Na (C-1) and NaCl 0.9% (C-2) to 1.48 ±0.27 and 1.78 ±0.22 respectively. The tissue edema tended to be lower in treatment groups T1 and T2, significantly decreasing to 1.12 ±0.18 and 1.05 ±0.13, respectively (Figures 2 A–C). However, the positive control group that had standard therapy of diosmin-hesperidin (C+) did not show a significant decrease compared to the C-1 group. This finding suggested that MSCs have a primary effect on tissue edema suppression. Furthermore, to identify the underlying mechanism of MSCs in hemorrhoids inhibition, we evaluated the molecular mechanism of MSCs in the inflammation pathway.
Figure 2
Tissue edema in hemorrhoid mouse model. A – Tissue edema (rectoanal coefficient) after induction with 6% croton oil. B – Representation of 20 mm resected rectoanal tissue C – mean tissue edema (rectoanal coefficient). Healthy: healthy group; C-1: negative control/hemorrhoid group, administered CMC-Na; C-2: negative control/hemorrhoid group, administered NaCl 0.9%; C+: positive control/standard therapy group, administration of diosmin-hesperidin; T1: MSC therapy group, administration of 1.5 × 106 cells; T2: combination therapy group, administration of MSCs + diosmin-hesperidin. Data are presented as mean ± SD with n = 5, *p < 0.03, **p < 0.002, ***p < 0.0002, ****p < 0.0001 indicates a significant difference
The combination of MSCs and diosmin-hesperidin treatment inhibited inflammation in hemorrhoids by dysregulation of TNF-α
The pathological process of hemorrhoids is characterized by the presence of inflammatory signs, such as tissue damage, blood vessel congestion, bleeding, and migration of inflammatory cells. Single MSC treatment (T1) and the combination of MSCs with diosmin-hesperidin (T2) significantly reduced the degree of inflammation in the croton oil-induced hemorrhoids model. The inflammation grade (both groups) was lower than that for standard therapy (C+) (Figures 3 A, B). The mechanism of MSCs reducing the inflammation in hemorrhoids was observed under TNF-α protein expression. The presence of MSCs dramatically reduced the TNF-α protein expression to 0.35 ±0.08 (Figures 3 C, D).
Figure 3
Rectoanal histology of croton oil-induced hemorrhoids under HE staining (A) and quantification of inflammation degree (B). C – Band intensity of TNF-α and b-actin in Western blot analysis. D – Quantification of TNF-α expression ratio to β-actin. Healthy: healthy group; C-1: negative control/hemorrhoid group, administered CMC-Na; C-2: negative control/hemorrhoid group, administered NaCl 0.9%; C+: positive control/standard therapy group, administration of diosmin-hesperidin; T1: MSC therapy group, administration of 1.5 × 106 cells; T2: combination therapy group, administration of MSCs + diosmin-hesperidin. Data are presented as mean ± SD with n = 5, *p < 0.03, **p < 0.002, ***p < 0.0002, ****p < 0.0001 indicates a significant difference
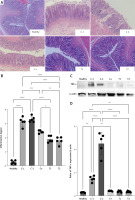
MSC treatment increased TGF-β and collagen type I/III ratio of croton oil-induced hemorrhoids
Transforming growth factor (TGF)-β is an important growth factor that plays a role in stimulating fibroblasts to proliferate into myofibroblasts that express α-smooth muscle actin (SMA) in the wound area, increasing fibronectin synthesis and collagen deposition in the proliferation phase. We found that MSCs as a single treatment or in combination with standard therapy increased the TGF-β protein expression more than a single standard therapy (Figures 4 A, B). Interestingly, the increase of TGF-β protein expression is in line with collagen type I/III ratio (Figures 4 C, D). This phenomenon confirms that the presence of MSCs accelerated the remodeling phase of wound healing in the hemorrhoids model. Thus, we confirmed this phenomenon by analyzing matrix metalloproteinase-9 (MMP-9), because the suppression of MMP-9 activity indicated the acceleration of inflammation stages into the remodeling stage.
Figure 4
A – Band intensity of TGF-β in Western blot analysis. B – Quantification of the TGF-β expression ratio to β-actin. C – Band intensity of collagen type I and collagen type III in Western blot analysis. D – Quantification of collagen type I/III ratio to b-actin. Healthy: healthy group; C-1: negative control/hemorrhoid group, administered CMC-Na; C-2: negative control/hemorrhoid group, administered NaCl 0.9%; C+: positive control/standard therapy group, administration of diosmin-hesperidin; T1: MSC therapy group, administration of 1.5 × 106 cells; T2: combination therapy group, administration of MSCs + diosmin-hesperidin. Data are presented as mean ± SD with n = 5, *p < 0.03, **p < 0.002, ***p < 0.0002, ****p < 0.0001 indicates a significant difference

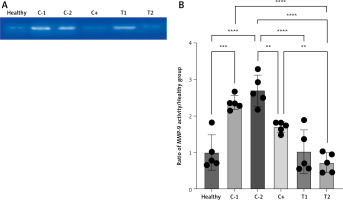
The presence of MMP-9 decreased the MMP-9 activity
The MMP-9 enzyme activity increased in the negative control/hemorrhoid group (C-1 and C-2) and tended to be low in the treatment group (T1 and T2) and positive control group (C+). The lowest MMP-9 activity was found in the combination therapy group (0.72 ±0.27) (Figures 5 A, B).
Figure 5
A – Band intensity of MMP-9 activity in gelatin zymography analysis. B – Quantification of the MMP-9 activity ratio to healthy group. Healthy: healthy group; C-1: negative control/haemorrhoid group, administered CMC-Na; C-2: negative control/hemorrhoid group, administered NaCl 0.9%; C+: positive control/standard therapy group, administration of diosmin-hesperidin; T1: MSC therapy group, administration of 1.5 × 106 cells; T2: combination therapy group, administration of MSCs + diosmin-hesperidin. Data are presented as mean ± SD with n = 5, *p < 0.03, **p < 0.002, ***p < 0.0002, ****p < 0.0001 indicates a significant difference
Discussion
The repeated croton oil administration into the rectoanal area (up to 20 mm from the anal opening) for 30 s over three consecutive days in this study induced mucosal ulceration or even tissue necrosis [11]. Inflammation acts as a protective host response against cellular damage or pathogens in microvascularized tissue [35, 36]. Damage-associated molecular patterns (DAMPs), such as HMGB1, released by damaged cells, activate local-tissue residential macrophages, which polarize into M1 macrophages and synthesize inflammatory mediators, potentially leading to local tissue damage, functional disruption, and chronic inflammation-related diseases, if unresolved [37, 38]. The pathological changes in anal cushions in hemorrhoidal disease result from inflammation, characterized by hemorrhoidal plexus dilation in the rectoanal area with associated connective tissue damage [3, 7]. Mesenchymal stem cells (MSCs) have been studied for their anti-inflammatory properties and their potential in treating various inflammatory diseases [17, 39, 40]. Several previous studies have shown that MSCs can reduce pro-inflammatory cytokine release, increase the production of anti-inflammatory factors, and inhibit the activation of immune cells, making them a promising option for the treatment of inflammatory conditions [20, 27, 41, 42]. This study utilized MSCs due to their ability to migrate to injury sites and their paracrine role by secreting essential factors in their microenvironment in extracellular vesicles such as microvesicles, exosomes, or apoptotic bodies [23, 43, 44].
This study revealed that MSCs were effective in suppressing inflammation, demonstrated by a significant reduction in tissue edema in the treatment group (T1 and T2) compared to the hemorrhoid group (C-1 and C-2). This demonstrates the anti-inflammatory effects of MSCs by reducing interstitial edema during inflammation [45]. Additionally, our study found that the combination of MSCs with diosmin-hesperidin showed a synergistic effect in reducing tissue edema compared to either treatment alone, although this was not statistically significant. Moreover, our study found that MSCs significantly reduced inflammation degrees in the hemorrhoid model compared to the hemorrhoid group (C-1 and C-2). This finding is due to the decrease of inflammatory cell infiltration [46–48]. The combined therapy of diosmin-hesperidin with MSCs also exhibited a statistically significant synergistic effect in reducing inflammation compared to diosmin-hesperidin (C+) alone but did not significantly differ from the mesenchymal stem cell group (T1). MSCs have been found to inhibit chronic inflammation and promote tissue repair. In this study we evaluated it on day 9. On the 9th day, it is still considered in the late phase of inflammation, and some pro-inflammatory cytokines are still at high levels. Therefore, the observation on the 9th day is aimed at assessing the ability of MSCs to accelerate the inflammation phase. Furthermore, the administration of MSCs reduced the activity of MMP-9 during inflammation in the hemorrhoid model, similar to previous studies demonstrating decreased MMP-9 levels and activity with MSC therapy in ischemic stroke models [49]. The combination of diosmin-hesperidin with MSCs also exhibited a statistically non-significant synergistic effect in reducing MMP-9 activity compared to either treatment alone.
MSCs promoted wound healing by promptly terminating the inflammatory phase, as evidenced by increased expression of TGF-β in the treatment group (T1 and T2) compared to the hemorrhoid group (C-1 and C-2). This finding demonstrated the role of MSCs in initiating proliferation by inducing TGF-β expression [50]. The combined therapy showed non-statistically significantly higher TGF-β expression than either therapy alone. This research concluded that MSCs effectively curbed inflammation, exhibited better anti-inflammatory properties than diosmin-hesperidin alone, and demonstrated a potential synergistic effect in combination therapy, emphasizing their role in reducing edema, inflammatory cell infiltration, and MMP-9 activity, and enhancing TGF-β expression during hemorrhoidal disease.
Our study has a limitation in that we did not investigate the potential side effect of umbilical cord MSC administration. Hence, further studies with a larger sample size and more comprehensive follow-up are needed to examine and analyze the possible side effect of locally administered MSCs.
In conclusion, the anti-inflammatory effects of MSCs are mediated by the release of trophic and anti-inflammatory factors, which interact with immune cells and inhibit inflammatory responses. Furthermore, the use of MSCs in regenerative medicine has shown promising results in modulating inflammation and treating inflammatory disorders including hemorrhoids.