Introduction
Obstruction of the ureter may occur due to congenital, iatrogenic or other reasons. This can cause hydronephrosis in the early stage and can lead to cellular inflammation, necrosis and atrophy in the kidney tissue [1]. Treatment of upper urinary tract obstructions aims to resolve the obstruction and protect and/or to regain the functional reserve of the kidneys. But it is obvious that long lasting obstructions will cause a decrease in the functional reserve of the kidneys. Studies have shown that reactive oxygen species (ROS) are responsible for the ischemia-reperfusion (I/R) damage. ROS disturb structural elements of the tissue and may cause damage. Several studies have reported about (I/R) damage in various tissue samples and that this tissue damage may be decreased with antioxidant therapy [2, 3].
Zofenopril is a proline amino acid derived lipophilic angiotensin converting enzyme (ACE) inhibitor with a long lasting tissue penetration feature. Experimental studies have shown that it reduces cardiac and renal I/R damage with its antioxidant feature and has a supportive cardiovascular effect in myocardial infarction patients [4, 5]. Pheniramine maleate (PM) is an alkylamine derived H1 receptor antagonist and is believed to prevent the formation and/or secretion of ROS by adhesion on cell membrane due to its lipophilic feature [6]. In vitro studies have shown that PM achieves this effect through activated neutrophils. It is thought that this antioxidant effect may be of benefit by reducing the tissue damage [7].
The aim of our study was to evaluate the antioxidant effect of PM and zofenopril on the oxidative stress and histopathological changes by partial unilateral ureteral obstruction (PUUO).
Material and methods
Twenty-four 3-4 months old female Sprague-Dawley rats weighing between 150 and 250 g were used. All procedures on the animals were performed according to the conditions of the 1986 Strasbourg Universal Declaration on Animal Welfare in the Medical Scientific Experimental Research and Practice Center (MSERPC) of Ankara Education and Research Hospital with the approval of the local ethic committee (Protocol Number: 06.03.2014/274) under the control of veterinary physicians. The animals were sheltered in standard rat cages, 6 per cage, under MSERPC controlled conditions and were fed with pellet feed for rodents and tap water. Removal of animal excretions, food and water supply and cleaning of the cages were provided by experienced staff and the veterinary physician of the center.
Surgical preparation of partial unilateral ureteral obstruction
After induction of general anesthesia with xylazine (5–10 mg/kg; IM) (Rompun, Bayer-Istanbul) and ketamine hydrochloride (50–70 mg/kg; IM) and antibiotic prophylaxis with ampicillin (100 mg/kg; IM) the operation site was disinfected with 10% povidone iodine solution. A 2 cm midline longitudinal abdominal incision was made that permitted access to the right kidney, ureter, and psoas muscle. Partial UUO was accomplished by enveloping the lower right ureter in the psoas muscle with a single suture of 2-0 silk transfixing the muscle laterally and medially creating a muscular tunnel through which the ureteral segment was gently compressed. To produce a unilateral partial obstruction the psoas-implanting technique described in 1962 by Ulm and Josephson was adapted [8, 9]. In sham operated rats, the ureter was exposed but not obstructed. The incision was closed according to the anatomic layers and a warm wet dressing was put on the incision wound.
Rats were administered meperidine HCl 5–10 mg/kg i.p. for postoperative analgesia and they were fed after recovery from anesthesia. On the 15th postoperative day the abdominal incisions were opened, the ligated ureter was excised including the renal pelvis, simple nephrectomy was performed and blood samples were taken from the vena cava inferior [10]. Specimens were isolated in phenol and stored at +4°C for histopathological examination.
Study groups
Rats were divided into 4 groups:
Group 1 (sham), consisting of 6 rats. The surgical procedure described above was performed without the ureter obstruction step and right nephrectomy through the same incision was performed immediately after closing it.
Group 2 (control group), consisting of 6 rats. The surgical procedure mentioned above was performed. The rats did not receive any medication after surgery.
Group 3 (PUUO + pheniramine maleate group), consisting of 6 rats. Rats with partial unilateral ureteral obstruction were given PM 10 mg/kg/day i.p. for 14 days.
Group 4 (PUUO + zofenopril group), consisting of 6 rats. Rats with partial unilateral ureteral obstruction were given zofenopril 10 mg/kg/day i.p. for 14 days.
On the 15th day after the first operation the right nephrectomy procedure was performed in groups 2, 3, 4 through the abdominal incision. Tissue and blood samples were collected and biochemically evaluated in terms of total antioxidant status (TAS), total oxidant status (TOS), oxidative stress index (OSI) and paraoxonase (PON) parameters and histopathologically according to cellular morphology.
Histopathologic, immunohistochemical and biochemical evaluation
Hematoxylin-eosin staining
Tissue samples were fixed in 10% neutral buffered formalin for light microscopic examination. After the routine tissue preparation process, 5 μm sections were taken with a rotary microtome (RM 2135, Leica). Slides are placed into hematoxylin (01562E, Surgipath, Bretton, Peterborough, Cambridgeshire) and eosin (01602E, Surgipath, Bretton, Peterborough, Cambridgeshire). After routine tissue preparation the slides were then dried in air processed with xylene for gaining transparence for 30 min and covered with Entellan (UN 1866, Merck, Darmstadt, Germany) [11]. All sections of kidney samples were examined for tubular necrosis. Briefly, a minimum of 50 proximal tubules associated with 50 glomeruli were examined for each slide and an average score was obtained. Severity of lesion was graded from 0 to 3 according to the percentage of tubular involvement. Slides were examined and assigned for severity of changes using scores on a scale in which 0 denotes no change; grade 1 – changes affecting < 25% tubular damage (mild); grade 2 – changes affecting 25–50% of tubules (moderate); grade 3 – changes affecting > 50% of tubules (severe). A semiquantitative evaluation of renal tissues was accomplished by scoring the degree of severity according to previously published criteria [12]. Histopathological analysis was performed by the same blinded pathologist at Kirikkale University Medical Faculty Pathology Department.
Total antioxidant status (TAS)
TAS kit was used for the measurement of total antioxidant status. TAS level was determined using the method developed by Erel. The principle of the method was based on the chemical reduction of the of the blue-green colored ABTS radical into the colorless ABTS form by the antioxidants. For the measurement a spectrophotometer (Shimadzu UV 1700A) was used at 25°C. Serum TAS levels were calculated in mmol Trolox equivalent/l with the formula TAS = ([ΔAbs standard 1] – [ΔAbs sample])/([ΔAbs standard 1] – [ΔAbs standard 2]) [13].
Total oxidant status (TOS)
TOS levels were determined using a TOS kit which operates using a novel automated and colorimetric measurement method as previously described by Erel. The principle of the method is based on the oxidation of ferrous ion-chelate complex to ferric ions by the oxidants in the sample. The TAS measurement by the mentioned spectrophotometer was used and serum TOS levels were calculated with the formula TOS = ([ΔAbs serum]/[ΔAbs standard]) × 20 in μmol H2O2 equivalent/l [14].
Calculation of oxidative stress index (OSI)
The TOS : TAS ratio was used as the OSI. To perform the calculation, the unit of TAS, mmol Trolox equivalent/l, was converted to mmol Trolox equivalent/l, and OSI was calculated as follows: OSI = [TOS, mmol H2O2 equivalent/l]/[TAS, mmol Trolox equivalent/l] × 100 [14].
Paraoxonase (PON)
PON activity was measured with a commercial experimental kit (Relassay, Gaziantep, Turkey). PON measurement may be performed by presence or absence of NaCl and M–1 cm–1 enzyme activity was calculated by using the 18290 molar absorption index [15].
Statistical analysis
The statistical evaluation of the obtained data was performed with SPSS 21 (IBM Corp., Armonk, NY, USA) and SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) by an independent, dedicated biostatistician uninvolved in the study and blinded to treatment assignment, and the results are expressed as the median and interquartile range. Differences between groups were analyzed with ANOVA test (with post hoc Tukey test for unequal groups) and statistical significance was determined as p < 0.05.
Results
Biochemical findings and results
The mean values of TAS levels were statistically significantly higher in groups 3 and 4 (0.91 ±0.01 mmol Trolox equivalent/l and 0.98 ±0.03 mmol Trolox equivalent/l) compared to groups 1 and group 2 (0.68 ±0.08 mmol Trolox equivalent/l and 0.82 ±0.04 mmol Trolox equivalent/l) respectively (p < 0.001) (Table I). There was a statistically significant difference in the TAS values between groups 3 and 4 in favor of group 4 (p < 0.001). Comparison of TOS levels revealed a statistically significantly lower level in group 4 (13.82 ±2.51 μmol H2O2 equivalent/l) compared to group 1 (19.18 ±0.52 μmol H2O2 equivalent/l) (p < 0.001) and a lower level in groups 3 and group 4 (15.28 ±1.24 and 13.82 ±2.51 μmol H2O2 equivalent/l) respectively compared to group 2 (25.33 ±3.89 μmol H2O2 equivalent/l) (p < 0.001). By the results of the parameter OSI a lower level was observed in groups 3 and 4 (17.22 ±1.61 AU and 14.17 ±3.22 AU) respectively compared to groups 1 and 2 (28.82 ±4.26 AU and 30.97 ±3.97 AU) respectively (p < 0.001) (Table I). There was a statistically significantly difference in the OSI values between groups 3 and 4 in favor of group 4 (p < 0.001).
Table I
Biochemical results
Serum PON levels revealed a statistically significantly higher level in groups 3 and 4 (56.6 ±2.3 mUI/ml and 72.3 ±1.9 mUI/ml) respectively compared to groups 1 and 2 (42.8 ±1.4 mUI/ml and 33.2 ±2.5 mUI/ml) respectively (p < 0.001). Comparison of group 4 with group 3 revealed a statistically significant difference in favor of group 4 (p < 0.001).
Histopathologic findings and results
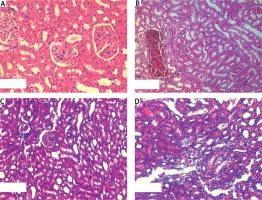
In the histopathologic evaluation of the study groups, glomerular atrophy, tubular degeneration, interstitial inflammation and congestion were observed less often in PM and in the zofenopril group compared to the control group (Figure 1). Scoring was done in the manner described by Ozbek et al. [12]. This decrease was more significant in the zofenopril group (Table II).
Discussion
Upper urinary tract obstructions (UUTO) can cause kidney loss. The duration and the level of the obstruction determine the treatment option. The first aim of the treatment is to resolve the obstruction, protect and/or partially regain the renal function. In long lasting severe obstruction the functional reserve of the kidney will decrease and result in total renal failure and nephrectomy. In recent literature there are many studies describing the ischemia/reperfusion damage in several tissues and stating that antioxidant treatment may reduce this condition [16, 17].
As the oxidative and antioxidative system is a complex formation, it may be misleading to measure and evaluate it only with one biomarker. We aimed to analyze different parameters at the same time in order to evaluate renal damage in the early stage in our study. The results indicating a protective effect in various organs in the former studies on zofenopril and PM encouraged us to think that they may have a renal protective effect by hydronephrosis due to ureter obstruction, which is very common in urological practice. Nosalova and Bayrak demonstrated the I/R damage preventive effect of pheniramine on the gastrointestinal and renal system respectively [7, 18]. Mak et al. detected that zofenopril has a protective effect against lipid peroxidation caused by the free radicals on endothelial cells [19]. Altunoluk et al. observed its protective effect against I/R damage by testis torsion [20].
Zofenopril is an antihypertensive drug and because of its common use by patients with heart diseases without any major side effects and its demonstrated beneficial effect in prior studies on experimental I/R on testis and heart, it may be of clinical use in patients with hydronephrosis due to ureter obstruction in order to protect renal function. Garrido et al. found that increased angiotensin-II levels enhance oxidative stress and Cacciatore et al. stated that ACE inhibitor treatment reduced oxidative stress [21, 22].
In our study PM and zofenopril biochemically decreased TOS and OSI. By comparison of these two drug groups with the control group it was revealed that zofenopril has a more potent effect than PM in the decline of the oxidative parameters (Table I). Histopathologic and immunohistochemical analyses revealed that PM and zofenopril decreased interstitial inflammation and congestion and this effect was observed as more significant in the zofenopril group (Table II). Also PON level, which is another indicator for antioxidant status, is observed to have decreased in the PM and zofenopril group (Table I).
It is certain that hydronephrosis will result in renal function loss. In this aspect, the time period under obstruction is of critical importance. Antioxidant agents may be useful in order to protect kidney function and reserves until the extinction of the obstruction and after [23–25]. Planning of future studies including renal function tests may bring addition of antioxidants into first line treatment options in order to protect the functions of the hydronephrotic kidneys.
The present study has some limitations. This is an experimental study using a relatively small number of animals from one strain, so the results may be limited to this species. It is not certain if the same results would be seen in different species, or in different models of kidney disease. This study may not represent much of the longer metabolic conditions that are exposed to pathology for a relatively short period of time. We believe that further experimental and clinical studies are needed for the mentioned clinical use of these two drugs.
In conclusion, the data from our study have shown that PM and zofenopril have antioxidant features by increasing TAS and decreasing TOS and OSI and PON levels, thus demonstrating upregulation as a systemic response in order to increase the TAS level. Histopathological examination revealed a more potent antiinflammatory effect in the zofenopril administered group. In the light of these results we consider that PM and zofenopril may have antioxidant and damage reducing effects by hydronephrosis due to partial ureteral obstruction.