Introduction
Congenital diaphragmatic hernia (CDH) is a developmental defect of the fetal diaphragm that allows the abdominal organs such as intestines, stomach, liver and spleen to move up into the thoracic cavity. CDH results in pulmonary hypoplasia and pulmonary hypertension [1, 2]. Most studies have assessed the overall mortality rate to range from 17% to 37% [3, 4]. The accurate prediction of outcome is an essential part of obstetric consultation with a pregnant woman carrying a fetus with CDH. High risk of neonatal death is the basis of eligibility for fetoscopic endotracheal occlusion (FETO) treatment. One of the most important prognostic factors, described by Metkus et al. in 1996, is the lung-to-head ratio (LHR), i.e. the measurement of the lung area opposite the hernia divided by fetal head circumference [5]. Currently it is recommended that the observed to expected LHR (o/e LRH) be used as an index of severity, i.e. the percentage of observed to expected LRH value for gestational age [6, 7]. A limitation of LHR measurement is that the interpretation of its value may vary significantly between different ultrasound users (interobserver error) [8, 9], which justifies the efforts to look for new prognostic markers. The authors of this study support the notion that evaluation of the fetal heart is a promising direction in the search for new prognostic parameters [10–14].
The purpose of the study was to evaluate whether selected prenatal markers from fetal echocardiography can predict postnatal outcome in CDH patients. We also aimed to verify the prognostic value of LHR in our series of cases.
Material and methods
Subjects
We examined 29 fetuses with CDH. The group included only those cases where the pregnancy was continued after prenatal diagnosis and a newborn was born alive. The patients included in the study received prenatal ultrasound diagnosis and were monitored in a reference center for fetal cardiology. Their births took place in the tertiary maternity unit between 2010 and 2020.
In 23 cases, CDH was an isolated defect. In 6 cases concomitant cardiac and extracardiac anomalies were present.
Of the 29 patients, 8 died before surgical repair. Surgical repair was performed in 18 patients, 8 of them survived until discharge, and 10 died in the postoperative period. Surgical repair data were missing for 3 patients; all of these patients died before discharge.
Method
An off-line quantitative retrospective study of selected, potentially prognostic parameters was performed based on digitized echocardiographic findings obtained in 29 fetuses with CDH. The group of 6 patients with concomitant cardiac and extracardiac anomalies was excluded from the further analysis, creating the group of 23 patients with isolated CDH. Patient data collected from hospital records included the details on perinatal period and treatment until the day an infant was discharged from hospital or died. We analyzed the following parameters measured using fetal echocardiography and ultrasound in the studied group:
Fetal heart size calculated by dividing the heart area by the chest area (HA/CA) (the four-chamber view).
Z-score for the main pulmonary artery (MPA) diameter based on gestational age of the examined fetuses (three-vessel view of the upper mediastinum).
Z-score for the ascending aorta (AAo) diameter based on gestational age of the examined fetuses (three-vessel view of the upper mediastinum).
Main pulmonary artery-to-ascending aorta ratio (MPA/AAo) (three-vessel view of the upper mediastinum).
Lung-to-head ratio (LHR) calculated as the lung area opposite the CDH divided by fetal head circumference. The lung area was measured by manual tracing of the lung borders (the tracing method).
Observed to expected lung-to-head ratio (o/e LHR), i.e. a percentage of the observed to expected value for gestational age.
The assessed parameters were compared between the group of patients with isolated CDH who survived to discharge and the subset of patients with isolated CDH who died before discharge. If ultrasound evaluation was performed multiple times in one fetus, only the most recent report was included in the analysis.
Statistical analysis
Mean values and standard deviation (SD) were measured for normally distributed continuous variables. Median and interquartile range (IQR) were measured for non-normally distributed continuous variables. Nominal variables were described with percentages. The t-test was used to compare normally distributed continuous variables between the subsets. Comparison of nominal variables was performed using the χ2 test. P < 0.05 was considered the threshold for statistical significance for all comparisons.
Results
Left-sided, right-sided, and bilateral CDH were diagnosed in 89.7% (26/29), 6.9% (2/29) and 3.4% (1/29) patients respectively. The abnormality was detected at mean gestational age of 23.6 ±6.4 weeks. The first fetal echocardiogram was performed at mean gestational age of 30.0 ±6.3 weeks. Prenatal tests showed normal cardiac anatomy in 79.3% (23/29) fetuses, whereas 20.7% (6/29) fetuses were diagnosed as having cardiac anomalies. In two fetuses with CDH and concomitant cardiac anomalies the presence of concomitant extracardiac anomalies was also detected (1 case with shortened fetal long bones and one with talipes equinovarus). Isolated CDH was found in 79.3% of patients (23/29). Fetoscopic endotracheal occlusion (FETO) was performed in 13.8% (4/29) of patients. The mean gestational age at delivery was 37.6 ±2.2 weeks. Forty-five percent (13/29) of patients were born vaginally and 55% (16/29) via C-section. The mean birth weight was 2946 ±528 g. Male and female infants constituted 54% (15/28) and 46% (13/28) of the group respectively (the data of 1 patient were missing). Most patients, i.e. 72.4% (21/29), died before discharge, whereas 27.6% (8/29) of patients survived beyond the neonatal period and were discharged from hospital. 34.8% (8/23) of patients with isolated CDH survived beyond the neonatal period.
Concomitant congenital heart disease
A concomitant congenital heart disease was prenatally diagnosed in 20.7% (6/29) of fetuses with CDH. The detected cardiac defects included:
isolated muscular VSD,
coarctation of the aorta (COA),
double outlet right ventricle (DORV) with left ventricular hypoplasia,
single ventricle with common arterial trunk,
complete atrioventricular canal (CAVC) with common arterial trunk,
CAVC with transposition of the great arteries (TGA) and pulmonary atresia.
Two of the patients with concomitant congenital heart disease also had concomitant extracardiac anomalies (1 case with shortened fetal long bones and 1 with talipes equinovarus).
None of the 6 patients with concomitant congenital heart disease survived beyond the neonatal period. In the group of patients with normal cardiac anatomy survival was 34.8% (8/23). The difference between the groups was not statistically significant (χ2, p = 0.09).
The group of 6 patients with concomitant cardiac and extracardiac anomalies was excluded from the further analysis.
Heart size
Four-chamber views of acceptable quality were obtained from 21 fetuses with isolated CDH included in the study, which allowed the assessment of heart area to chest area ratio (HA/CA). In the group of 8 patients who survived the neonatal period, mean HA/CA was 0.238 ±0.046. In the group of 13 patients who died before discharge, mean HA/CA was 0.236 ±0.043. The difference was not statistically significant (t-test, p = 0.47). In the group of 6 patients with HA/CA ratio ≤ 0.2, the survival was 33.3% (2/6). In the group of 15 patients with HA/CA ratio ≥ 0.2, the survival was 40% (6/15). The difference was not statistically significant (χ2, test p = 0.78).
Measurement of the great arteries
Nine fetuses were excluded from the analysis of AAo and MPA diameters due to incomplete imaging data on the measurements of the great vessels. The final sample consisted of 14 fetuses with isolated CDH (Table I).
Table I
Measurements of the great arteries – comparison of patients with CDH who survived beyond the neonatal period and CDH patients who died
| Parameter | Survivals | Deaths | T-test |
|---|---|---|---|
| MPA Z-score (mean) | 0.34 ±0.45 | –0.22 ±0.86 | p = 0.073 |
| AAo Z-score (mean) | 0.23 ±0.98 | –1.82 ±1.04 | p = 0.0015 |
| MPA/AAo | 1.22 ±0.17 | 1.46 ±0.21 | p = 0.017 |
In 6 patients who survived to discharge, mean z-score for MPA diameter was 0.34 ±0.45. In 8 patients who died before discharge, mean z-score for MPA diameter was – 0.22 ±0.86. The difference was not statistically significant (t-test, p = 0.073).
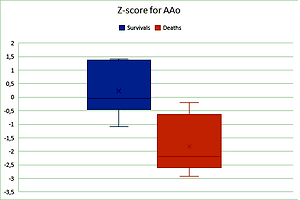
In 6 patients who survived to discharge, mean z-score for AAo diameter was 0.23 ±0.98. In 8 patients who died before discharge, mean z-score for AAo diameter was – 1.82 ±1.04. The difference was statistically significant (t-test, p = 0.0015) (Figure 1).
Figure 1
Z-score for AAo diameter was significantly higher in the group of 6 patients who survived than in the group of 8 patients who died (t-test, p = 0.0015)
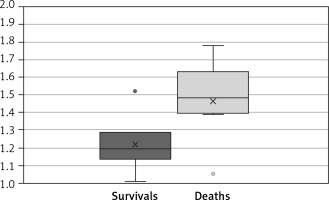
In the group of 6 patients who survived to discharge, the MPA/AAo ratio was 1.22 ±0.17. In the group of 8 patients who died before discharge, the MPA/AAo ratio was 1.46 ±0.21. The difference was statistically significant (t-test, p = 0.017) (Figure 2).
LHR parameter
Ultrasound images obtained from 17 fetuses with isolated CDH were of sufficiently good quality for an “off line” measurement of the LHR parameter and participation of the patients in the study. We analyzed the most recently obtained LHR values (median gestational age at the time of assessment was 33 weeks with an interquartile range (IQR) of 30 to 36 weeks).
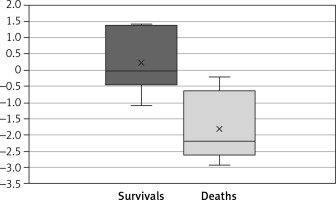
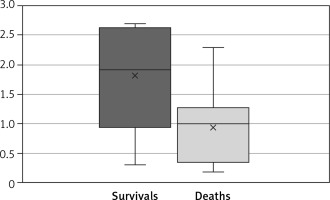
In the group of patients who survived to discharge, the LHR was 1.81 ±0.96. In the group of patients who died before discharge, the LHR was 0.95 ±0.6. The difference was statistically significant (t-test, p = 0.019) (Figure 3).
Figure 3
Mean LHR was significantly higher in the group of patients who survived than in the group of patients who died (t-test, p = 0.019)
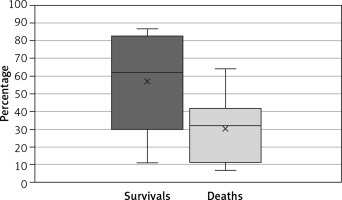
In the group of patients who survived to discharge, the o/e LHR was 57 ±30%. In the group of patients who died before discharge, the o/e LHR was 30 ±18%. The difference was statistically significant (t-test, p = 0.018) (Figure 4).
Discussion
In our research group small diameter of the AAo on the three-vessel view was associated with an increased neonatal mortality in the evaluated CDH fetuses. Another significant poor prognostic factor was a large disproportion between the size of the MPA and the AAo (in favor of the MPA). To the best of the authors’ knowledge, the association between small diameter of the AAo and mortality in patients with CDH has not previously been investigated. The earlier studies indicated that hypoplasia of the other left heart structures may be correlated with poor outcome in patients with CDH. An example of such an anatomical structure is the mitral valve. Yamoto et al. found that a ratio of tricuspid to mitral diameter greater than 1.72 was a highly specific and sensitive indicator of mortality risk in their patients. However, the authors found no significant correlation between MPA/AAo ratio and survival outcome [15].
A possible pathophysiological explanation for the correlation between hypoplasia of the aortic arch/left heart structures and increased mortality risk in patients with CDH is that these echocardiographic findings may indirectly indicate the severity of chest compression and altered hemodynamics associated with this anomaly. Underdevelopment of the left heart may be caused by compression of the fetal heart and lungs by herniated abdominal organs [16]. Pulmonary hypoplasia in CDH patients is associated with reduced pulmonary venous return from the lungs into the left atrium, which results in decreased filling of the LV and is likely to cause left heart hypoplasia [17]. Intrathoracic liver herniation is associated with alterations in the normal course of the ductus venosus and inferior vena cava, which may increase streaming toward the right atrium at the expense of the left atrium and subsequently cause left heart hypoplasia [18].
The above examples indicate that severity of narrowing of the ascending aorta may be indirectly associated with the severity of CDH. The research papers published to date analyzed prognostic indicators of survival in CDH patients, largely focusing on the MPA and its branches (left and right pulmonary artery LPA, RPA) rather than on the aorta.
Ruano et al. reported that in CDH patients who died in the neonatal period, MPA, LPA and RPA diameters were significantly smaller than those in CDH patients who survived [19]. Sokol et al. observed that reduced PA diameter at the site of the hernia as well as large disproportion between LPA and RPA correlate with increased mortality in patients with CDH. Severity of LPA/RPA hypoplasia indirectly reflected the severity of the pulmonary hypoplasia at the site of the hernia. The hypothesis was confirmed by autopsy [20]. Vuletin et al. analyzed MRI scans obtained from fetuses with left-sided CDH. They observed a correlation between reduced diameter of MPA branches and congenital pulmonary hypertension as well as poor prognostic outcome [21]. In our study group the mean MPA diameter was slightly larger in CDH patients who survived. However, the difference was not statistically significant.
In this study, we found no link between HA/CA value and survival prognosis in patients with CDH, despite the fact that reduced values of this parameter were considered potentially a valuable marker of the severity of heart and lung compression by the abdominal organs. Yamoto et al. produced different results which indicated a reduced HA/CA value in patients with CDH who died [15].
Of the group evaluated in this study, 24% of fetuses had CDH with concomitant congenital heart defect. None of these patients survived beyond the neonatal period. The association between concomitant congenital heart defect and increased CDH mortality is extensively documented in the medical literature. Respondek-Liberska et al. studied a group of 115 patients diagnosed with CDH and treated in a reference center in Poland between 1994 and 2006. Congenital cardiac anomalies were observed in 10% of the diagnosed fetuses, which also resulted in 100% mortality [14]. Więckowska et al. described a group of 6 infants with CDH and severe cardiac anomalies, diagnosed and treated in the same reference center between 2007 and 2017. None of the 6 patients lived beyond the neonatal period [22]. Cohen et al. reported that the risk of death was 2.9 times higher in fetuses with CDH and concomitant cardiac anomaly than in fetuses with isolated CDH. The mortality risk was significantly higher in patients with severe pulmonary hypoplasia, with a 100% mortality rate in fetuses with LHR < 1.2 [23]. Menon et al. reported that the mortality risk is indeed significantly higher in CDH patients with concomitant congenital heart defect. The mortality rates were the highest in patients with severe heart defects, such as TGA or HLHS. The authors emphasized the fact that the prognostic indicators of survival in patients with CDH and severe cardiac anomaly did not improve throughout the 10-year observation period [24].
Our findings confirm that LHR value is an important prognostic factor in patients with CDH.
Patients with CDH who died had significantly lower absolute LHR and o/e LHR values than CDH patients who survived. The correlation between low LHR values and high mortality in CDH patients was confirmed by multiple research papers. In a study by Lipshutz et al., all patients with LHR lower than 1.0 died, while all the other patients whose LHR was higher than 1.4 survived [25]. Alfaraj et al. estimated that for each unit decrease in o/e LHR, mortality increased by 11% [26]. A few studies have challenged the prognostic value of LHR. Arkovitz et al. found no correlation between LHR value lower than 1.0 and increased mortality odds or increased requirement for ECMO support [27].
We are aware that a limitation of our study is the fact that the mortality rate in our studied group is significantly higher than values reported by many other centers. Snoek et al. described a group of 122 neonates with isolated CDH diagnosed in the prenatal period. The survival rate in this group was 77.9%, while in our studied group it was only 34.8% [28]. It should be noted, however, that our studied group included more cases with the severe form of CDH. In the group of Snoek et al., cases of severe CDH (o/e LHR ≤ 25%) accounted for 3.3%, while in our group they represented 29.4% of all cases. We also emphasize that our studied group does not consist of all CDH cases from 2010–2020 treated in our center, but only those in which a fetal echocardiographic examination was performed in the reference center for fetal cardiology. Another limitation of our study may be the small number of patients in the studied group. CDH is a very rare defect (according to the US Centers for Disease Control and Prevention, the incidence is 2.6 per 10,000 births). Our study included only prenatally diagnosed cases with satisfactory fetal echocardiography performed. The above factors resulted in the small size of the research group. Nevertheless, our results regarding the prognostic value of narrow ascending aorta were statistically significant and may be a platform for a further, larger cohort that will assess our findings.
In conclusion, the findings of this study indicate that narrowing of the ascending aorta in CDH fetuses was a poor prognostic factor associated with increased mortality in neonates. The narrowing of the ascending aorta is reflected in the low z-score for AAo diameter and high MPA/AAo ratio. The above parameters are easily measurable via prenatal echocardiography and can be used in clinical practice. Our study also confirmed the prognostic value of both absolute and o/e LHR.