Introduction
Aging is a natural process involving dysfunction of multiple organs and is characterized by increased susceptibility to infections, cancer and autoimmune diseases [1–5]. In this regard, the immune response in aged individual is usually ineffective, inappropriate, and potentially harmful. The altered functionality of the immune system associated with aging has been termed immunosenescence, and is associated with an enhanced risk of severe infections, and a low efficacy of vaccination [6–10]. In addition, immunosenescence is associated with abnormalities in proportion of different leukocytes subsets in primary and secondary lymphoid organs, as well as defects in humoral and cellular immune responses [11, 12]. Moreover, thymus involution begins at early aging, and aged mice have shown a significant decrease in development of T lymphocytes [13, 14], with a progressive reduction of the proportion of naïve CD4+ T cells. In contrast, CD8+ T lymphocytes usually remain without any alteration in elderly men [15–17]. Immunocompetence in aging men has been assessed in vivo to recall antigens by delayed-type hypersensitivity tests [18] as well as antibodies production upon vaccination [19, 20]. In addition, several in vitro studies have shown a poor T cell capacity to proliferate in response to specific antigens or mitogenic stimuli [21, 22]. Furthermore, T lymphocytes from aging men have displayed short telomeres [23, 24], restricted T cell receptor repertoire [25], diminished capacity to synthesize IL-2 and reduced expression of CD25 upon in vitro stimulation [22, 26].
On the other hand, telomeres are specialized molecular complexes at the end of chromosomes, consisting of tandem hexanucleotide sequences (5’-TTAGGG-3’) and other proteins called shelterins such as telomere repeat binding protein factors 1 and 2 (TRF1 and 2), and protection of telomere (POT1), etc. [27]. In humans, telomere length ranges from 10 to 20 kb, and a shortening occurs during each cellular division. When telomere length reaches a critical point (4–5 kb) it may trigger cell cycle arrest, cellular senescence or apoptosis. Genetic and epigenetic factors, sex, hormones, cellular stress and inflammation are involved in telomere shortening, and an inverse correlation between telomere length and aging has been widely reported [28–30].
On the other hand, clonal expansion of T and B-cell is necessary for an adequate immune response, and the ability of these cells to proliferate is highly dependent on telomere shortening. Interestingly, lymphocytes are able to up-regulate activity of telomerase, an enzyme that stabilize the telomeres during the process of DNA replication and cell division, increasing cellular survival [31]. Thus, telomere shortening in T lymphocytes could be inhibited by several factors including IL-7 and IL-15, which increase telomerase activity or induce the expression of proteins members of the Bcl-2 family [32, 33]. Interestingly, telomere attrition could be used as a marker for reduced proliferative reserve in hematopoietic progenitor cells [34].
Interleukin-2 receptor is structured by α (CD25), β (CD122), and γ (CD132) chains, which interact with IL-2 to regulate differentiation, function and homeostasis of several effector T-cell subsets; mainly CD4+ Th1, and CD8+ cytotoxic lymphocytes. Signaling through IL-2R plays a critical role regulating the adaptive immune system and controlling T cell proliferation and survival. The above mechanisms are required for maintenance of immune tolerance and cellular homeostasis [35–37]. Therefore, telomere dynamics is critical in maintaining immune cell function, immunosenescence and pathogenesis regulation of several diseases such as autoimmunity.
However, the possible relation between IL-2, CD25, telomere shortening and the proliferation capacity of T lymphocytes in aging has not been completely elucidated.
The aim of this study was to determine the possible association of telomere length with IL-2 production, CD25 expression and proliferation capacity of T lymphocytes in aging men.
Material and methods
Antibodies and reagents
The following mouse anti-human monoclonal antibodies were purchased from BD Biosciences (San Diego, CA): anti-CD3 APC (HIT3a), anti-CD25 PE (M-A251), anti-CD4 PerCP (SK3), and anti-CD8 FITC (HIT8a). An IL-2 secretion assay detection kit was obtained from R&D Systems, Inc. (Quantikine ELISA, Minneapolis, MN), and carboxyfluorescein succinimidyl ester (CFSE) was purchased from Molecular Probes (Eugene, OR).
Subjects
Blood samples were obtained from twenty healthy men from 20 to 25 years old (young men group), and 20 men from 60 to 65 years old (aged men group). All individuals included in this study were healthy and individuals with any clinical disease manifestation, smokers or those with alcohol or drug consumption were excluded. A standardized questionnaire was applied which included questions regarding personal medical history. Anthropometric measurements were taken by a standardized method. The blood pressure was taken using a mercury sphygmomanometer with the subjects seated for 10 min prior to measurement. To determine the body mass index (BMI) values, a daily calibrated digital scale and stadiometer were used to measure body weight and height (Table I). This protocol was approved by the local bioethics committee and written informed consent was obtained from each volunteer.
Isolation of mononuclear cells, CFSE dilution and in vitro cell stimulation assays
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation (Sigma-Aldrich, St. Louis, MO). PBMCs were loaded with cell tracker dye CFSE (0.5 μM; Molecular Probes) to monitor proliferation. Cells were adjusted at a concentration of 1 × 106/ml in RPMI-1640 tissue culture medium (GIBCO, Eugene, OR) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 U/ml penicillin, and 50 mg/ml streptomycin. Finally, CFSE-labeled PBMCs were cultured with or without 2.5 μg/ml of concanavalin A (Con A, Sigma Aldrich) for 72 h at 37ºC, 100% humidity and 5% CO2. At the end of cell culture, cell proliferation (by CFSE dilution) and CD25 expression were determined by flow cytometry on CD3+ gated cells.
Flow cytometry analysis
To determine the expression of CD25 on T lymphocytes, freshly isolated peripheral blood mononuclear cells were stained with anti-CD8 FITC, CD25 PE, CD4 PerCP and CD3 APC. In all cases, cells were analyzed with a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA) using the Cell Quest software (Becton Dickinson). Also, for the histograms, the region of negativity (auto fluorescence) was calculated using a fluorescent control isotype. The lymphocytes were gated using forward and side scatter to exclude debris and dead cells, and 10,000 events were acquired in each assay.
Measurement of IL-2 in cell culture supernatant
Interleukin-2 was quantified in supernatant from unstimulated and Con A-stimulated PBMCs from each individual, using the Quantikine ELISA kits (R&D Systems) according the manufacturer’s recommendations.
Telomere length measurement
Total DNA was isolated from PBMCs before and after in vitro stimulation with Con A with phenol-chloroform. We performed a pico-green quantitation using a Molecular Devices 96-well spectrophotometer and results were confirmed using a NanoDrop SD-1000 spectrophotometer. Relative average telomere length was assessed by a modified version of the real-time PCR-based telomere assay previously reported by Cawthon [38]. The telomere length (kb) was determined using a RT-PCR assay (LightCycler, Roche) to generate the standard curve for each run and to determine the dilution factors of standards corresponding to T and S amounts in each sample. Briefly, the telomere repeat copy number to single gene copy number (T/S) ratio was determined using a RT-PCR assay (LightCycler, Roche). The following primers were used for tel 1, 5’-G2T5GAG3TGAG3TGAG3TGAG3TGAG3T-3 and tel 2,3’-TC3GACTATC3TATC3TAT-C3TATC3TATC3TA-5’. The constitutive gene primers 36B4u: 5’-CAGCAAGTG3A2G2TGTA2TCC-3’ and 36B4d: 3’-C3AT2CTACATCA2CG3TACA2-5’ were used as a control. For telomere determination the reaction conditions were as follows: 1 cycle at 95°C for 5 min, followed by 40 cycles at 95°C for 15 s, 54°C for 60 s, and 72°C for 28 s; and for the gene 36B4, 1 cycle at 95°C for 5 min, followed by 40 cycles at 95°C for 15 s, 58°C for 20 s, and 72°C for 28 s. All samples for both telomeres and 36B4 were run in triplicate; and the threshold value for both reactions was set to 0.5. Additionally, to assess and compensate intra-assay variations in PCR efficiency, a four-point standard curve from 12.5 to 100 ng using genomic DNA was prepared.
Statistical analysis
The Mann-Whitney U and Wilcoxon’s tests were used to determine significant differences between two groups. In addition, the Spearman correlation test was used to analyze the degree of association between two variables. To analyze the relation between length telomere and T cell proliferation, Fisher’s exact test was used. Statistical analysis was performed using the PASW Statistics 18 software.
Results
Telomere length in PBMCs before and after Con A stimulation
As expected, in freshly isolated PBMCs shorter telomeres were detected in aged men compared to young men (6.38 ±0.06 and 12.42 ±0.08 kb, respectively, p < 0.05, Figure 1). When lymphocytes were in vitro stimulated with Con A, cells from young men tended to increase their telomere length upon mitogenic stimulation, whereas cells from aged men did not (Figure 1 A). No difference was detected between telomere length of fresh and non-stimulated PBMCs (data no shown). Because Con A in vitro stimulation induces non-specific T lymphocyte proliferation, a slight increase in the proportion of CD3+ lymphocytes in young men, but not in aged men, was observed (Figures 1 B and 2 B), displaying to CD3+ T lymphocytes as proliferating cells (Figure 2 B). While in young men the proportion of CD3– cells showed a slight decrease after Con A stimulation, it was not observed in aged men (Figure 1 B).
Figure 1
Telomere length in peripheral blood mononuclear cells (PBMCs) before and after Con A stimulation. The telomere length in PBMC from young or aged men was measured by RT-PCR in fresh cells and after in vitro Con A stimulation. Graph in A corresponds to median telomere length in kilobase (kb) in PBMCs from twenty young and twenty aged men. *p < 0.05 by Wilcoxon test. In B representative flow cytometry histograms are shown the proportions of CD3+ (M2) and CD3– (M1) PBMCs from young (upper panels) and aged subjects (lower panels), without stimulus (fresh cells) or after Con A stimulation. The lymphocytes were gated using forward and side scatter to exclude debris and dead cells, and 10,000 events were acquired in each assay

Figure 2
T cell subsets after in vitro stimulation. PBMCs from young or aged men were cultured with or without Con A for 72 h. Then, cells were stained with anti-CD3, CD4, and CD8 monoclonal antibodies and analyzed by flow cytometry. A – Represents the arithmetic mean and SD of the percentage of non-stimulated (white bar) or Con A in vitro stimulated CD3+ lymphocytes (black bar). B and C – Represent the arithmetic mean and SD of the percentage of non-stimulated or Con A in vitro stimulated CD4+ and CD8+ cells gated on CD3+, respectively. *p < 0.05 by Mann-Whitney U test

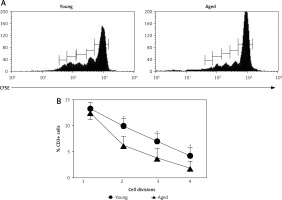
Cell division after in vitro T cell stimulation
Freshly isolated PBMCs were in vitro stimulated with Con A and proliferation of CD3+ cells was determined. In most cases, a higher percent of PBMCs reached more divisions in young men than in aged men (Figure 3 A). Correspondingly, CD3+ lymphocytes were the proliferating cells in young men (Figures 2 A and 3 B). Likewise, a significant increase in the percentage of CD3+ CD4+ T cells was observed upon stimulation with Con A in young men, but not in aged men (Figure 2 B). Accordingly, a significant decrease in the percentage of CD8+ cells was observed in young men, while no difference in CD8+ cells was detected in aged men (Figure 2 C).
Figure 3
Cell division after in vitro T cell stimulation. The CFSE-labeled peripheral blood mononuclear cells (PBMCs) were cultured with or without Con A for 72 h and the percentage of divided cells was determined by flow cytometry. Panel A corresponds to representative flow cytometry histograms of CFSE dilution on Con A stimulated cells gated on CD3+ and panel B corresponds to the arithmetic mean and SD of percentages of cells reaching 1, 2, 3 and 4 cell divisions, respectively
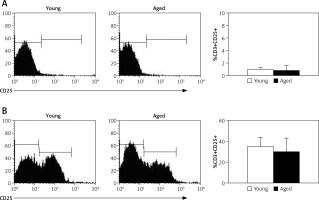
CD25 expression and IL-2 production by CD3+ lymphocytes
To explore the possible role of IL-2 on CD3+ lymphocyte proliferation, both the expression of CD25 on CD3+ lymphocytes and IL-2 production were determined upon in vitro stimulation with Con A. Thus, in non-stimulated cells, low levels of CD25 were detected on CD3+ lymphocytes from both young and aged men (Figure 4 A). After in vitro stimulation with Con A the percentage of CD3+CD25+ tended to be higher in young men, but no significant difference was detected with respect to aging men (Figure 4 B). Likewise, similar levels of CD25 expression were observed on CD4+ and CD8+ lymphocytes (data no shown).
Figure 4
CD25 expression on CD3+ lymphocytes. PBMCs from young or old subjects were cultured without stimuli (A) or with Con A (B) for 72 h and the percentage of cells expressing CD25 was determined by flow cytometry. Left and central panels correspond to representative flow cytometry histograms of CD25 expression gated on CD3+ cells, and right panel represents the arithmetic mean and SD of the percentage of CD3+CD25+ cells
On the other hand, IL-2 levels in cell culture supernatants increased upon Con A stimulation in both groups. However no significant differences in levels of this cytokine were detected in young and aged men (Table II).
Table II
Levels of IL-2 in the cell culture supernatants of non-stimulated and Con A stimulated PBMCs. PBMCs from young or aged subjects were cultured with or without Con A for 72 h, and the synthesis of IL-2 was quantified by ELISA. The results correspond to the arithmetic mean and SD of IL-2 in pg/ml. The levels of IL-2 in the cell culture supernatants increased upon Con A stimulation, and no significant differences in the synthesis of this cytokine were detected between young and aged men, by Mann-Whitney U test
| IL-2 [pg/ml] | Young | Aged | P-value |
|---|---|---|---|
| Medium | 6 ±5.6 | 6 ±5.1 | 0.66 |
| Con A | 44.6 ±10 | 66.5 ±9.5 | 0.22 |
Relation of T cell proliferation capacity and telomere length in young and aged men
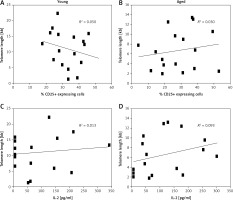
Above we have shown that a higher percent of CD3+ lymphocytes reached more divisions in young men than in aged men (Figure 3). Also, we detected shorter telomeres in cells from aged men compared to young men, on both freshly isolated cells and after Con A stimulation, respectively (Figure 1). Therefore, we analyzed the relation between telomere length and the proliferation capacity of CD3+ T lymphocytes. For this, we considered the percentage of subjects in whom cells have reached ≥ 3 cell divisions, and a cut-off of 3 cell divisions was considered as a low proliferation capacity. Also a rate of ≤ 6 kb was considered as a shorter telomere length. Using Fisher’s exact test, our data show a direct relation between low proliferation capacity (≤ 3 cell divisions) and shorter telomere length (≤ 6 kb), in aged men (Figure 5). In addition, in both aged and young men no significant correlation was detected between the levels of CD25 expression (Figure 6 A, B) or IL-2 synthesis and telomere length (Figure 6 C, D).
Figure 5
Relation of T cell proliferation capacity and telomere length in young and aged men. The percentage of cases in which the cells have reached ≤ 3 or ≥ 3 divisions is shown. A value of ≤ 3 has been considered as a lower proliferation capacity. Also a rate of ≤ 6 kb was considered as a shorter telomere length. Using Fisher’s exact test, our data shown a direct relation between low proliferation capacity (3 divisions) and a significant telomere length shortening ≤ 6 kb (p = 0.02)
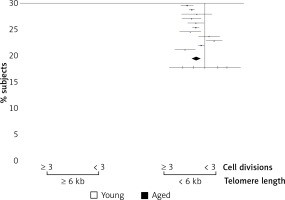
Figure 6
Statistical correlation between levels of CD25 expression or IL-2 synthesis and telomere length. A and B show correlations between telomere length and percentage of CD25 expressing cells. In C and D correlations between telomere length and IL-2 production (pg/ml) are shown. The squares represent individual data of each subject; in the left panels are young men, while in the right panels are aged men. Spearman’s test was used to analyze the association between two variables
Discussion
Immunosenescence is characterized by several immunological alterations, which are associated with increased risk of infections, cancer and autoimmunity. The functionality of the immune system depends on the lymphocytes’ capacity to respond to any antigenic challenge and induce clonal expansion. Thus, the telomere shortening and telomerase activity in lymphocytes seem to have an important role in the capability of the immune system to generate an immune response. However, studies of telomere attrition and cellular mechanisms of the immune system are uncertain and controversial.
Because the proliferation of T cells is dependent of both IL-2 and the expression of its high affinity receptor CD25, we decided to assess the possible relation between the reduced capacity of T lymphocytes from aged men to proliferate and CD25 expression as well as in vitro production of IL-2. As previously reported by others [21, 24, 39], we detected that PBMCs from aged men have shorter telomeres as well as a significantly reduced proliferation capacity of T cells. However, Peres et al. [17] did not observe differences in mitogen-induced proliferation of lymphocytes from young and old individuals. Similar results were reported by Wallace et al. [40]. It is feasible that these discordant results may be due to the subjects’ age in the studied groups, the number of individuals included, and cell culture conditions used in each study.
It is well known that telomere length may be influenced by sex, hormones, reactive oxygen species, as well as by genetic and epigenetic factors. Thus, to control the influence of sexual hormones, only healthy men were included in our study. Therefore, telomere length shortening and reduced capacity of T cell proliferation observed in aged men could be attributed merely to aging, because these alterations were not observed in young men. However, it is worth mentioning that blood pressure tended to be higher in aged men included in our study compared to young men. Thus, our results might also be influenced by this phenomenon, since it has been reported that telomere length is affected by arterial hypertension and cardiovascular disease [41, 42].
It is important to note that we evaluated the telomere length in PBMCs purified from fresh whole blood by Ficoll-Hypaque density gradient centrifugation. Most monocytes and granulocytes are excluded with this procedure. Therefore, telomere length measurement corresponds to T and B lymphocytes, which are present in a greater proportion in purified PBMCs. Furthermore, it was referred to as the T lymphocyte subset, because the stimulus used in our experiments was Con A. Con A is a lectin that induces non-specific T cell proliferation, but has no effect on B cells. Consequently, upon PBMC in vitro Con A stimulation, the cells on which the telomere length could be modified by cell division are T cells. As we expected, fresh PBMCs or non-stimulated cells (data not shown) from aged subjects had shorter telomeres than cells from young men. Moreover, when lymphocytes from aged subjects were induced to undergo in vitro proliferation with Con A, increased shortening of telomeric DNA was observed, whereas in cells from young men it was not. On the other hand, it is feasible that cells from young men increased the level and activity of telomerase upon mitogenic stimulation, because an increase in telomere length was detected. Telomerase is an enzyme that stabilizes the telomeres in the process of DNA replication and cell division, extending the capacity for replication and inducing the expression of some anti-apoptotic proteins [23, 31]. In this regard, it has been reported that aging subjects have a high percentage of memory T cells, which bear short telomeres, and have shown a decreased ability to respond to antigen recall [43, 44]. Therefore very low levels or low activity of telomerase could be associated with shorter telomeres as well as reduced capacity of T cell proliferation in aged men. Likewise Yang et al. [45] observed the relation between telomere length and cellular proliferation as a function of donor age. They found that cells from adrenal tissue cultured in vitro from donors of different ages showed a strong age-related decline in total replication capacity.
On the other hand, IL-2 is an important growth and differentiation factor for T cells [36, 37], and it has been shown that lymphocytes from aged individuals show a diminished capability to synthesize IL-2 and defective expression of CD25 [22, 26]. However, our results showed that CD25 expression on CD3+ lymphocytes and in vitro production of IL-2 were similar in cells from young and aged men. These results are in agreement with previous reports showing no apparent differences in the function of the IL-2/CD25 axis in cells from young and aged subjects [17]. However, it is necessary to carry out further studies to explore the intracellular pathways induced through IL2-CD25 interaction in cells from aged men.
It is clear that age-related changes in telomere length of PBMCs in vivo occur at different rates in different individuals and cell types, revealing that changes in the length of telomeres in T cells with aging are influenced by several factors, such as telomerase activity and health conditions [46]. Although our study has some limitations such as having measured the telomere shortening in total PBMCs and not in purified T cell subsets, the fact that there is a deficiency of CD3+ T cell proliferation after in vitro stimulation in aging, although the expression levels of CD25 and IL2 production were similar between young and aged individuals, suggests that the responsiveness of CD3+ T lymphocytes is due to the inability to proliferate by telomere shortening and not by the IL2-CD25 interaction. As mentioned above, future studies are needed to test this hypothesis. Additionally, it is well known that in vitro Con A stimulation strongly induces non-specific T cell proliferation. It is worth highlighting that in this study a low dose (2.5 μg/ml of Con A) was used to prevent overstimulation-induced cell death; despite the low proliferation index shown here, we were able to detect differences in lymphocyte proliferation capacity between young and aged men.
In conclusion, our data confirm that cells from aged men had a short telomere length and a restricted capability to proliferate; however, these phenomena are not related to defects in either IL-2 production or CD25 expression on CD3+ T lymphocytes.