Introduction
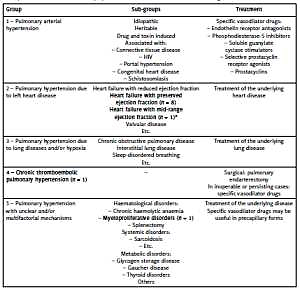
A sub-group of myeloproliferative neoplasms (MPN) is BCR-ABL1-negative MPN that includes polycythemia vera, essential thrombocythemia, and primary myelofibrosis [1]. These disorders are characterized by stem cell-derived clonal myeloproliferation. Although the association between MPN and precapillary pulmonary hypertension (PH) has been suggested by case reports and small series, the exact prevalence of pulmonary vascular abnormalities in myeloid diseases is currently not known [2–9]. Pulmonary hypertension represents a heterogeneous group of disorders that are classified based on differences in clinical, hemodynamic, and histopathologic features. In the general population the most common forms of PH are associated with left heart (LH) disease (Group 2) or with pulmonary diseases (Group 3). Pulmonary arterial hypertension (PAH – Group 1) is a progressive disorder characterized by remodelling of the small pulmonary arteries, resulting in increased pulmonary vascular resistance [10]. In the context of MPN, typical forms of PH are chronic thromboembolic PH (CTEPH – Group 4), drug-induced PH, and a characteristic precapillary PH with multifactorial origin (Group 5). The latter form may mimic Group 1 PAH [10, 11]. Pulmonary hypertension due to LH disease is classified as postcapillary as it is characterized by elevated left ventricular filling pressure. The other groups comprise precapillary or mixed PH-forms. Treatment of PH is strictly determined by the type of the disease. If it is caused by LH disease or lung diseases, our choice is to optimize the therapy of the underlying condition. Group 2 and 3 patients should not routinely be treated with vasoactive agents approved for the treatment of PAH [10]. A modified version of the current classification of PH and group-specific treatment options are reported in Table I [10].
Table I
Modified version of the current classification of pulmonary hypertension (PH) [10]. Group-specific treatment options are also presented. Type and number of PH cases found in our myeloproliferative neoplasms population are formatted in bold (*The definition of heart failure with mid-range left ventricular ejection fraction was first reported in the literature in 2016 [17]; therefore it is not mentioned in the recent PH guideline)
Once precapillary PH is diagnosed, the prognosis of MPN patients is unfavourable [7, 8]. Echocardiography enables a clinically satisfactory differential diagnosis between pre- and postcapillary PH [12, 13]. As primary screening tool, however, echocardiography is not feasible due to its limited availability and high cost. For this reason, there is a need for reliable biomarkers for screening of precapillary PH in MPN patients. N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a useful but not specific marker of right ventricular dysfunction of PH patients [14–16] as its level is also elevated in patients with LH failure [17] and/or atrial fibrillation [18, 19]. Vascular endothelial growth factor (VEGF) – one of the signal proteins stimulating angiogenesis – may be another potential biomarker as its elevated serum levels have been reported in patients with idiopathic PAH [20, 21]. On the other hand, decreased VEGF values have been reported in patients with LH disease [22, 23]. In addition, VEGF is believed to contribute to the pathogenesis of MPN [24] and it has also been suggested that VEGF may play a causative role in induction of Group 5 precapillary PH in MPN patients [5, 25].
Thus the aim of this study was to determine whether combined use of NT-proBNP and VEGF as biomarkers is suitable for the detection of the precapillary forms of PH in a large MPN population.
Material and methods
Patients
Outpatients with MPN were consecutively screened into this prospective cohort study during a seven-month period in the Department of Haematology, Somogy County Kaposi Mór Teaching Hospital. Considered as a pilot study, a convenient sample size of 90 patients was chosen. Inclusion criteria were: diagnosis of MPN based on the current algorithms [1], age ≥ 18 years, capacity to give informed consent, stable disease (no transformation into acute myeloid leukaemia). Exclusion criteria were: refusal to give informed consent, poor echo window. Baseline clinical (age, gender, body mass index (BMI), JAK2 positivity) and laboratory (blood count, estimated glomerular filtration rate (eGFR), uric acid level) data, as well as data about medication and co-morbidities were collected. The study complied with the Declaration of Helsinki. The institutional ethics committee approved the study. Informed consent was obtained from all individual participants included in the study.
Echocardiography
12-lead surface electrocardiogram (ECG) was obtained to diagnose atrial fibrillation. Echocardiography was performed using the Philips IE330 ultrasound system (Philips Healthcare, Best, The Netherlands) by a single investigator. Left ventricular ejection fraction was measured by biplane Simpson’s method. Left ventricular mass was calculated according to the Devereux formula. As a parameter of right ventricular systolic function, tricuspid annular plane systolic excursion (TAPSE) was measured [26]. In addition to the parameters of mitral inflow (peak of the early (E) and late (A) diastolic velocities), myocardial early (eʹ) diastolic velocities were measured at the lateral and septal border of the mitral annulus by pulsed tissue Doppler. Lateral and septal eʹ values were averaged. Mitral E/A and E/eʹ ratios were calculated. Left ventricular diastolic function as well as severity of the aortic, mitral , and tricuspid regurgitations were classified according to the current recommendations [27, 28]. Systolic pulmonary artery pressure (sPAP) was estimated as the sum of the pressure difference across the tricuspid valve calculated using the modified Bernoulli equation and an estimate of mean right atrial pressure using the diameter and the collapsibility index of the inferior vena cava (IVC) (5 mm Hg if IVC ≤ 21 mm and collapse with sniff > 50%; 15 mm Hg if IVC > 21 mm and collapse with sniff < 50%; 10 mm Hg at intermediate values) [29]. Pulmonary hypertension was defined as Doppler-derived sPAP ≥ 40 mm Hg. Differential diagnosis between pre- and postcapillary PH was based on echocardiographic data. Characteristics of the precapillary PH were as follows: dilated right heart, systolo-diastolic D-sign, dilated IVC without inspiratory collapse, left ventricular E/e’ < 10 [11].
NT-proBNP and VEGF assays
Blood samples were collected on the day of the echocardiography. Analysis of plasma VEGF levels was performed using human VEGF-A Platinum ELISA kit (eBioscience, San Diego, CA, USA). NT-proBNP was measured using electrochemiluminescence immunoassay on the Elecsys 2010 system (Roche Diagnostics, Mannheim, Germany).
Statistical analysis
Continuous variables are presented as mean ± SD. Categorical variables are expressed as frequencies and percentages. Comparisons between groups were performed using the independent Mann-Whitney test for NT-proBNP and VEGF levels. Since NT-proBNP and VEGF values did not show a normal distribution, logarithmic transformation was performed (lnNT-proBNP, lnVEGF). Clinical variables that correlate with NT-proBNP and VEGF levels were determined using bivariate Pearson correlation. Receiver-operating characteristic (ROC) curve analysis was used to examine the performance of NT-proBNP in predicting cardiopulmonary involvement (precapillary PH, PH due to LH disease, left ventricular ejection fraction < 50%, atrial fibrillation) and the performance of VEGF in predicting LH disease (PH due to LH disease, left ventricular ejection fraction < 50%, atrial fibrillation). The latter analysis was repeated using the ratio of serum VEGF to platelet count as a biomarker [30, 31]. Optimal cut-off values were chosen to maximize sensitivity and specificity. A p-value of < 0.05 was considered significant. Data were analysed using SPSS 22.0 statistical software (SPSS, Chicago, Illinois).
Results
Ninety consecutive patients were evaluated for inclusion. After exclusion of 9 patients (2 refused to sign the informed consent; in 1 patient MPN transformed to overt acute myeloid leukaemia and in 6 patients echocardiographic data could not be obtained due to unfavourable thoracic anatomy) 81 patients were enrolled in the study – 39 (48%), 39 (48%) and 3 (4%) patients had essential thrombocytosis, polycythemia vera and primary myelofibrosis, respectively. Clinical and laboratory data of the 81 patients are outlined in Table II.
Table II
Clinical and echocardiographic data of the myeloproliferative neoplasms (MPN) patients and their correlations with NT-proBNP (ln)
| Factor | MPN patients (n = 81) | Correlation with NT-proBNP (ln) | |
|---|---|---|---|
| r | P | ||
| Age [years] | 67.1 ±12.5 | 0.509 | < 0.001 |
| Female gender, n (%) | 51 (63) | 0.130 | 0.248 |
| Body mass index [kg/m2] | 27 ±5 | –0.213 | 0.155 |
| JAK2 positivity, n (%) | 66 (81.5) | 0.020 | 0.862 |
| Disease duration [years] | 6.2 ±4.5 | 0.048 | 0.670 |
| Splenomegaly, n (%) | 14 (17) | 0.120 | 0.284 |
| Recent smoker, n (%) | 11 (14) | 0.112 | 0.318 |
| Laboratory data: | |||
| Haemoglobin [g/l] | 138.4 ±19.6 | –0.263 | 0.018 |
| White blood cells [109/l] | 8.6 ±3.4 | 0.016 | 0.891 |
| Platelet [109/l] | 427.1 ±207.6 | –0.130 | 0.246 |
| eGFR [ml/min/1.73 m2] | 70.3 ±19.9 | –0.515 | < 0.001 |
| Uric acid [μmol/l] | 339.4 ±82.2 | 0.378 | 0.001 |
| NT-proBNP [pg/ml] | 532.1 ±1195.1 | – | |
| VEGF [pg/ml] | 789.0 ±737.6 | –0.021 | 0.855* |
| Medication, n (%): | |||
| Acetylsalicylic acid | 55 (68) | –0.123 | 0.274 |
| Hydroxyurea | 69 (85) | 0.075 | 0.506 |
| Anagrelide | 17 (21) | 0.056 | 0.617 |
| PEG-IFN-2α | 15 (19) | 0.104 | 0.356 |
| Ruxolitinib | 4 (5) | 0.015 | 0.892 |
| Co-morbidities, n (%): | |||
| History of thromboembolic event | 19 (23) | 0.036 | 0.753 |
| Systemic hypertension | 58 (72) | 0.017 | 0.883 |
| Diabetes | 12 (15) | 0.035 | 0.755 |
| Atrial fibrillation | 7 (9) | 0.414 | < 0.001 |
| Echocardiographic data: | |||
| LV ejection fraction (%) | 62.5 ±6.6 | –0.325 | 0.004 |
| sPAP [mm Hg] | 32.8 ±10.9 | 0.550 | < 0.001 |
| E/A | 0.8 ±0.3 | –0.397 | 0.001 |
| Left ventricular mass [g] | 222 ±59.3 | 0.163 | 0.146 |
| Mitral regurgitation (mild: moderate: severe), n | 36 : 19 : 5 | 0.329 | 0.036 |
| Tricuspid regurgitation (mild: moderate: severe), n | 48 : 14 : 2 | 0.349 | 0.004 |
| Aortic regurgitation (mild: moderate: severe), n | 16 : 5 : 0 | 0.082 | 0.723 |
| TAPSE [mm] | 27.4 ±3.6 | –0.402 | < 0.001 |
| Average mitral annular eʹ [cm/s] | 7.5 ±2.6 | –0.314 | 0.014 |
| Mitral E/eʹ | 9.7 ±3.8 | 0.310 | 0.016 |
| IVC [mm] | 19.9 ±3.7 | 0.031 | 0.919 |
In 4 (5%) patients moderately reduced (35–50%) left ventricular ejection fraction was found. Further echocardiographic data are reported in Table II.
Pulmonary hypertension was diagnosed in 11 (13.6%) cases (Table I): In 8 patients PH was associated with heart failure with preserved left ventricular ejection fraction (HFpEF) while in 1 patient it was associated with heart failure with mid-range left ventricular ejection fraction (HFmrEF). Two (2.5%) patients showed the typical signs of precapillary PH, so they underwent further investigation. In the first patient, pulmonary embolism and lung parenchyma diseases were excluded. Precapillary PH (Group 5) was diagnosed. In the second patient clear evidence of CTEPH was found. Detailed characteristics of these patients are outlined in Table III.
Table III
Detailed characteristics of our myeloproliferative neoplasms patients with precapillary pulmonary hypertension
[i] sPAP – systolic pulmonary artery pressure, RHC – right heart catheterization, PH – pulmonary hypertension, ET – essential thrombocythemia, PV – polycythemia vera, HU – hydroxyurea, RBBB – right bundle branch block, CTEPH – chronic thromboembolic pulmonary hypertension, PAWP – pulmonary artery wedge pressure, CO – cardiac output, PVR – pulmonary vascular resistance.
Atrial fibrillation was found in 7 (9%) patients, including the patient with HFmrEF-PH and 3 of those with HFpEF-PH.
NT-proBNP levels significantly correlated with the non-invasively measured sPAP values, and with several other clinical and echocardiographic parameters (Table II). Significantly higher NT-proBNP values were found in patients with cardiopulmonary involvement (left ventricular ejection fraction < 50%, PH due to LH disease, precapillary PH, atrial fibrillation; n = 15) than in the other MPN patients (1891.5 ±2214.6 vs. 223.1 ±422.2 pg/ml; p < 0.001). Extremely high NT-proBNP values were found in both patients with precapillary PH (Table III). Using ROC analysis, NT-proBNP ≥ 466 pg/ ml was the best predictor of cardiopulmonary involvement (AUC: 0.962, sensitivity: 86.7%, specificity: 93.9%).
No correlation was found between VEGF levels and sPAP values. Among all clinical and echocardiographic parameters, VEGF levels showed a significant correlation only with platelet number (r = 0.277, p = 0.014). VEGF values were lower in patients with LH disease (left ventricular ejection fraction < 50%, PH due to LH disease, atrial fibrillation; n = 13) compared with the rest of the population (534.7 ±676.0 vs. 823.1 ±738.6 pg/ml; p = 0.214), but the difference was not statistically significant. Using ROC analysis, VEGF ≤ 431 pg/ml was the best predictor of LH disease (AUC: 0.609, sensitivity: 76.9%, specificity: 62.7%). VEGF level was higher in our Group 5 patient, while it was lower in our CTEPH patient than this cut-off value (Table III). The diagnostic performance of the test improved only mildly if the ratio of serum VEGF to platelet count was used as a biomarker (AUC: 0.641).
Discussion
The possible association between precapillary PH and MPN has been suggested by case reports and small case series [2–9]. Nevertheless, the exact prevalence and incidence of the pulmonary vascular involvement are unknown in this disease. In the study of Chebrek et al. low prevalence of precapillary PH was observed (less than 5%) in MPN patients [32]. This result is quite different from that given by analysis of data from the previous literature, which revealed a global prevalence of 38% [2–5]. This discrepancy may have different explanations. First, the small size of the series reported may represent a bias in the study. In addition, the rate of precapillary PH may be greatly overestimated by echocardiography without careful elimination of patients with left ventricular dysfunction.
Due to its restricted size and the single-centre design, our study was not adequately powered to assess the prevalence of precapillary PH in MPN patients. Our data suggest, however, that PH due to LH disease is relatively common in this elderly, multimorbid population while precapillary forms of PH are less frequent.
Three major distinct clinical forms of PH have been described in patients with MPN: CTEPH, drug-induced PH and precapillary PH with multifactorial origin (Group 5). Although MPN – especially polycythemia vera and essential thrombocytosis – is a thrombophilic state, the epidemiological and clinical data on CTEPH in MPN patients are scarce. In their retrospective study Guilpan et al. reported six MPN patients with CTEPH. Interestingly, the diagnosis of CTEPH and MPN was made simultaneously in all patients, suggesting that CTEPH may be the first manifestation of the myeloproliferative diseases [6].
Several factors may contribute to precapillary (Group 5) PH in MPN. One possible explanation for the pathogenesis of PH is enhanced angiogenesis [21]. Group 5-PH is usually diagnosed late: 8-13.5 years after the recognition of the myeloproliferative disorder [6, 7]. The effectiveness of PAH-specific vasodilator therapy has not been proven yet in MPN. The prognosis is poor; the median time to death is 18 months after the diagnosis [8].
Tyrosine kinase inhibitors are used as a therapy for chronic myeloid leukaemia. Several case reports have suggested that PH may be one of the potential complications of dasatinib. Although a few patients have experienced full clinical and haemodynamic recovery after discontinuation of dasatinib, the majority have not recovered completely [33].
In our study, 2 patients showed clear signs of precapillary PH. Dasatinib use was not documented in these patients. Unfortunately our CTEPH patient refused invasive evaluation and further treatment and died within 6 months. In our Group 5-PH patient combined pulmonary vasodilator treatment was introduced. 28 months later the patient is still alive, in a New York Heart Association II–III functional status. Her history suggests that the use of the PAH-specific vasodilator drugs is worth further investigation in Group 5-PH patients.
Although according to the guidelines the final diagnosis of precapillary PH requires right heart catheterization (RHC), echocardiography is able to differentiate between post- and precapillary PH with good accuracy [12, 13]. Nevertheless, as a primary screening tool, the use of biomarkers seems to be more feasible. Thus we aimed to evaluate whether combined use of two biomarkers – NT-proBNP and VEGF – is suitable for detection of the precapillary forms of PH in MPN patients.
NT-proBNP is a neurohormone secreted mainly in the cardiac ventricles in response to volume expansion or pressure overload. Its prognostic power has been reported in various conditions [34, 35]. Its diagnostic and prognostic value was also proved in PH [16, 36]. In special populations with high risk for the development of PAH, NT-proBNP has been suggested as a sensitive biomarker for screening [37, 38]. Nevertheless, elevated NT-proBNP is not specific for right-sided heart disease, as its level is also elevated in patients with LH failure [17] and/or atrial fibrillation [18, 19]. Several other confounding factors may also raise the level of this biomarker: advanced age, anaemia, or renal dysfunction [39, 40]. Despite the well recognizable effects of these factors, NT-proBNP showed good diagnostic performance in identifying patients with manifested cardiopulmonary involvement in our study. It is of note that especially high NT-proBNP values were found in both patients with precapillary PH. Still, MPN patients with elevated NT-proBNP values require further investigation (ECG and echocardiography) to identify the type of their cardiopulmonary involvement.
VEGF plays a critical role in angiogenesis due to its ability to regulate proliferation, migration, specialisation and survival of endothelial cells [41]. In bone marrow of BCR-ABL1-negative MPN patients increased microvessel density and VEGF expression were reported [42, 43]. Furthermore, VEGF has been reported to be a key contributor to angiogenesis and vasculogenesis in idiopathic PAH, where elevated pulmonary artery pressure is the consequence of vasoconstriction and remodelling of pulmonary microvasculature [20, 21, 44]. Similarly, VEGF may play a causative role in induction of Group 5 PH in MPN patients [6, 25]. Data about the VEGF level of CTEPH patients are contradictory [20, 21], while decreased VEGF values have been reported in patients with LH disease [22, 23]. In our study VEGF showed a clear tendency to be lower in patients with LH disease than in the rest of the population. Our results suggest, however, that VEGF is not a suitable screening tool for this purpose due to its moderate sensitivity and low specificity. The correlation between VEGF level and platelet count has already been reported in the literature [45, 46]; thus the actual platelet count may affect the diagnostic abilities of this biomarker. Nevertheless, the use of the ratio of serum VEGF to platelet count did not improve the diagnostic performance substantially in our study. Similarly to the results of Suzuki et al. [20], a low VEGF value was found in our CTEPH patient. The high VEGF level of our Group 5 PH patient is in line with the results of Cortelezzi et al. [5]. Further studies are required involving numerous MPN patients with Group 5 PH to investigate whether VEGF plays a central role in the pathogenesis of this special condition.
Some limitations of our study are to be acknowledged. First of all, due to its restricted sample size the correlations between biomarkers and other variables may be misclassified.
Due to the lack of a healthy control group we could not define the cut-off value between normal and elevated/reduced VEGF levels.
Although severe precapillary PH is rare in chronic obstructive pulmonary disease (COPD) [47] and elevation of natriuretic peptide levels is typically mild [48], presence of COPD may influence the NT-proBNP level of the patients. In addition, COPD may have an impact on the VEGF levels [49]. Pulmonological data, however, were not available in our MPN population.
Several confounding factors may influence the velocity of the tricuspid regurgitation, including anaemia and consequential high cardiac output; therefore RHC may be mandatory for the correct diagnosis and classification of PH. In our study, however, RHC was performed in only one of the patients with precapillary PH.
In conclusion, NT-proBNP levels reflected cardiopulmonary involvement with high accuracy, but the combination of NT-proBNP and VEGF was not suitable for detection of the precapillary PH in our MPN population, as the diagnostic power of VEGF was limited. Highly elevated NT-proBNP levels may suggest precapillary PH, but further investigation is necessary for the exclusion of LH disease and/or atrial fibrillation.