Sanguiin has the functions of hemostasis and cooling blood, clearing away heat and toxin, astringent and antidiarrheal activity, and inhibiting a variety of pathogenic microorganisms and tumors. It can treat hematemesis, dysentery, burns, eczema, upper gastrointestinal bleeding, ulcer bleeding, hematochezia, metrorrhagia, tuberculous abscess, chronic osteomyelitis and other diseases [1, 2]. Based on animal models, this research proposed possible therapeutic targets and studied the mechanisms to make a reference for clinical treatment in cerebral hemorrhage animal models.
Results
The effect of Sanguiin on brain edema and behavior after cerebral hemorrhage in rats
According to the magnetic resonance imaging (MRI) results, there was no significant difference in the hematoma size and degree of edema between the drug administration group and the vehicle group before the administration (12 h). After the administration (72 h), there was no significant change in the volume of the hematoma between the two groups, but the edema in the vehicle group progressed significantly, which was heavier than that in the Sanguiin group. By measuring the brain swelling index, it was found that the increase of the brain swelling index in the vehicle group was significantly higher than that in the Sanguiin group. Through the determination of brain water content, it was found that 72 h after cerebral hemorrhage, the brain water content of the affected hemisphere was significantly higher than that of the sham group, and the intervention of Sanguiin can significantly reduce the increase in the cerebral water content of the affected side, suggesting that Sanguiin can effectively reduce the degree of brain edema after cerebral hemorrhage, as shown in Supplementary Figure S1.
On the 3rd day after cerebral hemorrhage, the rats showed neurological deficits in behavior. The results of the mNSS scoring and forelimb placement test showed that the drug had an effect of improving the neurological deficit caused by cerebral hemorrhage. In the corner experiment, Sanguiin significantly improved the neurological deficit of cerebral hemorrhage rats, as shown in Supplementary Figure S2.
The protective effect of Sanguiin on the blood-brain barrier after cerebral hemorrhage in rats
The EB penetration test showed that there was almost no extravasation of EB outside the capillaries in the sham group, but EB exudation was obviously increased after cerebral hemorrhage. The drug can improve this situation, which is consistent with the results of EB fluorescence. EB quantification also showed that Sanguiin can reduce EB extravasation, suggesting that Sanguiin can alleviate blood-brain barrier damage caused by cerebral hemorrhage, as shown in Supplementary Figure S3.
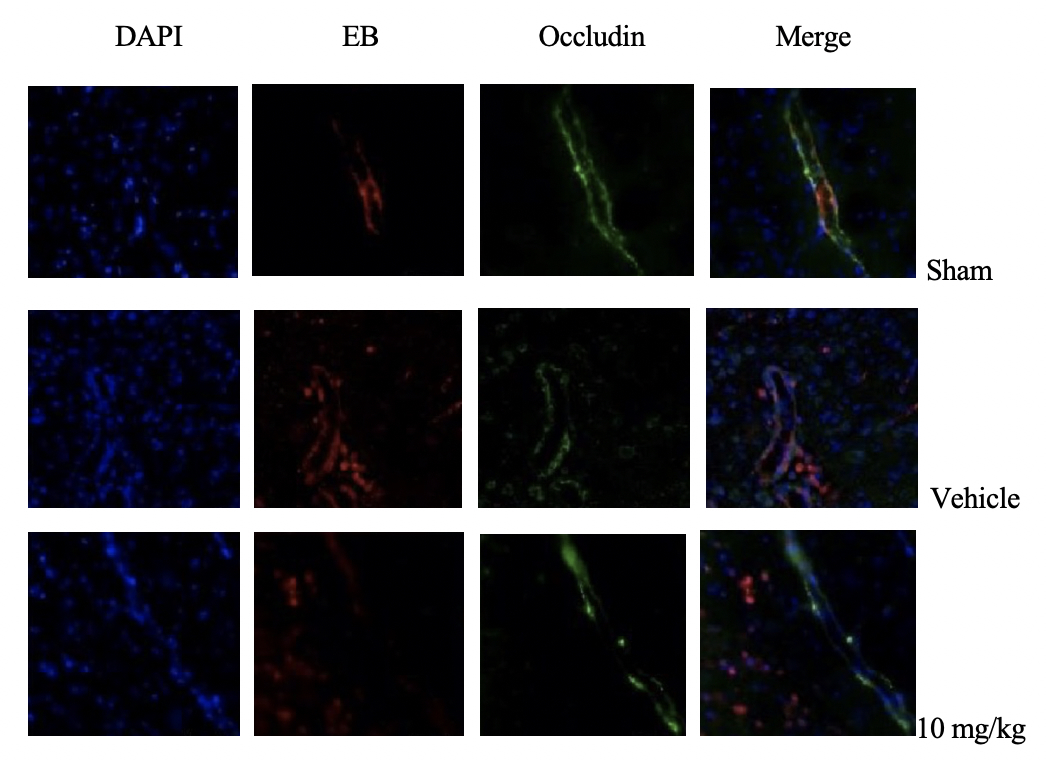
The blood-brain barrier-related tight junction proteins Zonula occludens-1 (ZO-1) and Occludin were labeled, and it was found that in the sham group, after perfusion, there was almost no EB dye residue in the brain tissue, while in the drug administration group and the vehicle group there was leaked EB dye residue out of the blood vessels. The expressions of ZO-1 and Occludin were discontinuous with EB expression, indicating that the blood-brain barrier was damaged after cerebral hemorrhage. The damage of tight junction protein in the drug administration group was less than that in the vehicle group, suggesting that Sanguiin had a certain protective effect on the blood-brain barrier after cerebral hemorrhage (Figure 1).
WB results also confirmed that the expressions of ZO-1 and Occludin decreased after cerebral hemorrhage, and Sanguiin improved this situation. In addition, matrix metalloproteinase-9 (MMP-9) is the tight junction protein that destroys the blood-brain barrier after cerebral hemorrhage. However, Sanguiin can down-regulate the expression of MMP-9 after cerebral hemorrhage, as shown in Supplementary Figure S4.
Discussion
Globally, the annual incidence of cerebral hemorrhage is 24.6 per 100,000, accounting for 10% to 15% of all types of strokes. The fatality rate in the first year can exceed 30%, and more than 80% of the survivors have neurological dysfunction in varying degrees, which seriously affects the quality of life of patients and becomes an urgent public health problem [3, 4]. The mechanism of brain edema formation after cerebral hemorrhage mainly includes two parts: angioedema and cytotoxic edema, which are affected by blood clot contraction, thrombin and coagulation cascade, red blood cell lysis and hemoglobin release [5–7].
Cerebral edema is almost inevitable after primary cerebral hemorrhage. It can form rapidly after hemorrhage, and rapidly increase the volume around the hematoma by about 75%, which seriously affects the prognosis of patients [8–10].
Under the action of hydrostatic pressure gradient and osmotic pressure, the liquid enters the surrounding parenchyma from the hematoma and capillaries, forming edema [11]. In this process, the red blood cells in the hematoma area maintain structural integrity and do not affect the edema process [12]. Besides, thrombin can also induce neuroinflammation, cause glial cell apoptosis near the hematoma, aggravate blood-brain barrier damage, and promote the development of cerebral edema [13, 14]. In addition, edema after cerebral hemorrhage is also related to hypoxia, complement activation, neuroendocrine regulation and other mechanisms induced by hematoma space-occupying [15–17].
In the study, we first used the rat collagenase-induced cerebral hemorrhage model to observe the cerebral edema, behavioral changes and blood-brain barrier-related changes in rats after treatment with Sanguiin [18–20]. The mechanism may be related to the protective effect of Sanguiin on the blood-brain barrier: decreasing the expression of matrix metalloproteinases, up-regulating tight junction protein content, and stabilizing the permeability of the blood-brain barrier.
In conclusion, Sanguiin can significantly improve the neurological deficits in rats with cerebral hemorrhage, and down-regulate the expression of MMP-9 after cerebral hemorrhage, suggesting that Sanguiin has a certain protective effect on the blood-brain barrier after cerebral hemorrhage.