Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / SYSTEMATIC REVIEW/META-ANALYSIS
Safety of denosumab versus zoledronic acid in patients with bone-related diseases: a systematic review and meta-analysis
1
Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
2
Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
3
Department of Orthopedic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Submission date: 2021-03-24
Final revision date: 2021-08-04
Acceptance date: 2021-09-16
Online publication date: 2021-09-17
Corresponding author
Wenhao Luo
Department of Orthopedics Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Post code: 510282, Beijing, 100730, China
Department of Orthopedics Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Post code: 510282, Beijing, 100730, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The aim of the study was to compare the safety of denosumab (Dmab) versus zoledronic acid (ZA) in patients with bone-related diseases. Both Dmab and ZA have been widely used in the treatment of bone-related diseases, but which drug is an optimal treatment in terms of safety remains controversial.
Material and methods:
PubMed, Embase, Web of Science, the Cochrane Central Library, and ClinicalTrials.gov were systematically searched up to 1st January 2021, and were evaluated by Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Randomized controlled trials comparing relevant outcomes of Dmab versus ZA in patients with bone-related diseases were included.
Results:
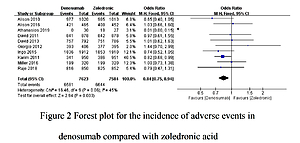
A total of 13 studies involving 21,042 participants were included. The incidence of total adverse events was significantly lower in patients receiving Dmab treatment than in those undergoing ZA treatment (OR = 0.84, 95% CI: 0.75–0.94, p = 0.003). Nine trials comparing Dmab with ZA further indicated that Dmab was significantly better than ZA in controlling the incidence of serious adverse events (OR = 0.91, 95% CI: 0.85–0.99, p = 0.02). Compared to ZA, Dmab administration was correlated with a lower risk of skeletal-related events (OR = 0.77, 95% CI: 0.70–0.85, p = 0.00001). However, no significant difference was found in the rate of infection events between Dmab and ZA (OR = 1.06, 95% CI: 0.93–1.20, p = 0.39).
Conclusions:
This study demonstrated superiority of Dmab over ZA in treating bone-related diseases in terms of safety and efficacy.
The aim of the study was to compare the safety of denosumab (Dmab) versus zoledronic acid (ZA) in patients with bone-related diseases. Both Dmab and ZA have been widely used in the treatment of bone-related diseases, but which drug is an optimal treatment in terms of safety remains controversial.
Material and methods:
PubMed, Embase, Web of Science, the Cochrane Central Library, and ClinicalTrials.gov were systematically searched up to 1st January 2021, and were evaluated by Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Randomized controlled trials comparing relevant outcomes of Dmab versus ZA in patients with bone-related diseases were included.
Results:
A total of 13 studies involving 21,042 participants were included. The incidence of total adverse events was significantly lower in patients receiving Dmab treatment than in those undergoing ZA treatment (OR = 0.84, 95% CI: 0.75–0.94, p = 0.003). Nine trials comparing Dmab with ZA further indicated that Dmab was significantly better than ZA in controlling the incidence of serious adverse events (OR = 0.91, 95% CI: 0.85–0.99, p = 0.02). Compared to ZA, Dmab administration was correlated with a lower risk of skeletal-related events (OR = 0.77, 95% CI: 0.70–0.85, p = 0.00001). However, no significant difference was found in the rate of infection events between Dmab and ZA (OR = 1.06, 95% CI: 0.93–1.20, p = 0.39).
Conclusions:
This study demonstrated superiority of Dmab over ZA in treating bone-related diseases in terms of safety and efficacy.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.