Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
GASTROENTEROLOGY / RESEARCH PAPER
STAT1/MUC4 activation promotes antimicrobial peptide production to reduce intestinal epithelium barrier injury caused by enteropathogenic Escherichia coli infection
1
The First Hospital of Qinhuangdao, China
Submission date: 2023-05-04
Final revision date: 2023-08-31
Acceptance date: 2023-09-01
Online publication date: 2023-09-03
KEYWORDS
ZO-1antimicrobial peptideenteropathogenic Escherichia coli infectionintestinal epithelium barrier injurySTAT1/MUC4 pathway
TOPICS
ABSTRACT
Introduction:
Antimicrobial peptides (AMPs) are endogenous peptides that have been identified to alleviate intestinal epithelial barrier inflammation and dysfunction caused by enteropathogenic Escherichia coli (EPEC) infection; nonetheless, the upstream molecular mechanism of the production of AMPs is poorly understood.
Material and methods:
The binding of signal transducer and activator of transcription (STAT) 1 (STAT1) to mucin 4 (MUC4) was examined by Co-Immunoprecipitation assay. To detect the influence of STAT1 and MUC4 expression, C57BL/6 mice model of EPEC infection in vivo and EPEC infected intestinal epithelial cells (IEC) in vitro model was established. Expressions of STAT1, MUC4, phosphorylated (p)-STAT1, proinflammatory cytokines, zonula occludens-1 (ZO-1) and AMP-related genes in mouse ileum and/or IEC were analyzed by immunohistochemical test, immunofluorescence assay, Western blot, and/or qRT-PCR. Meanwhile, IEC viability and apoptosis were measured using CCK-8 assay and flow cytometry.
Results:
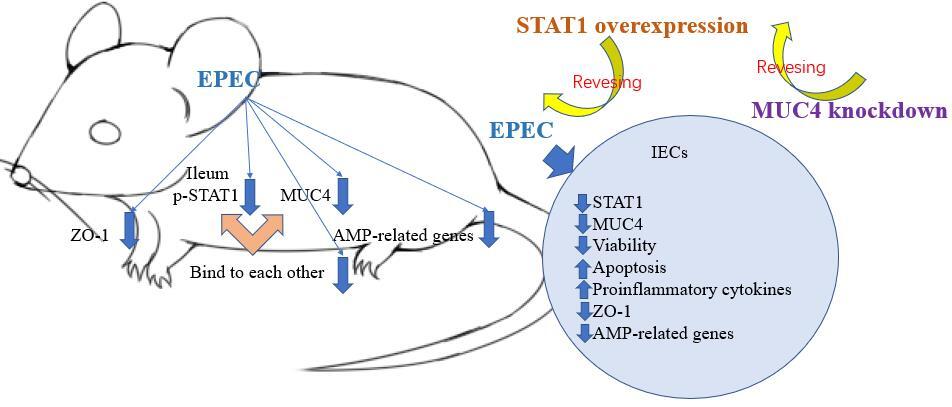
p-STAT1, MUC4, ZO-1 and AMP-related gene were lowly expressed in the ileum of EPEC-infected mice. p-STAT1 and MUC4 bound to each other. The expressions of STAT1 and MUC4 were decreased in EPEC-infected IEC. Overexpression of STAT1 resisted EPEC-induced viability decrease, apoptosis promotion, ZO-1 activity inhibition, release of proinflammatory cytokines, and downregulation of MUC4 and AMP-related genes in IEC. MUC4 knockdown partly counteracted the effect of STAT1 overexpression, but did not affect the forced STAT1 overexpression in EPEC-infected IEC.
Conclusions:
STAT1/MUC4 pathway activation promotes AMP production to mitigate intestinal epithelium barrier injury caused by EPEC infection.
Antimicrobial peptides (AMPs) are endogenous peptides that have been identified to alleviate intestinal epithelial barrier inflammation and dysfunction caused by enteropathogenic Escherichia coli (EPEC) infection; nonetheless, the upstream molecular mechanism of the production of AMPs is poorly understood.
Material and methods:
The binding of signal transducer and activator of transcription (STAT) 1 (STAT1) to mucin 4 (MUC4) was examined by Co-Immunoprecipitation assay. To detect the influence of STAT1 and MUC4 expression, C57BL/6 mice model of EPEC infection in vivo and EPEC infected intestinal epithelial cells (IEC) in vitro model was established. Expressions of STAT1, MUC4, phosphorylated (p)-STAT1, proinflammatory cytokines, zonula occludens-1 (ZO-1) and AMP-related genes in mouse ileum and/or IEC were analyzed by immunohistochemical test, immunofluorescence assay, Western blot, and/or qRT-PCR. Meanwhile, IEC viability and apoptosis were measured using CCK-8 assay and flow cytometry.
Results:
p-STAT1, MUC4, ZO-1 and AMP-related gene were lowly expressed in the ileum of EPEC-infected mice. p-STAT1 and MUC4 bound to each other. The expressions of STAT1 and MUC4 were decreased in EPEC-infected IEC. Overexpression of STAT1 resisted EPEC-induced viability decrease, apoptosis promotion, ZO-1 activity inhibition, release of proinflammatory cytokines, and downregulation of MUC4 and AMP-related genes in IEC. MUC4 knockdown partly counteracted the effect of STAT1 overexpression, but did not affect the forced STAT1 overexpression in EPEC-infected IEC.
Conclusions:
STAT1/MUC4 pathway activation promotes AMP production to mitigate intestinal epithelium barrier injury caused by EPEC infection.