Introduction
According to the World Health Organization (WHO) chronic obstructive pulmonary disease (COPD) is one of the most dangerous killers worldwide and ranks 3rd in the list among all the diseases. It has been estimated that more than 5 million peoples will be affected in the next 3 decades [1]. Chronic bronchitis, constricted airway remodeling and emphysema are the main features of COPD, but the main factor contributing to COPD-mediated deaths is the remittance of acute inflammation episodes [2–5]. Recently smoking of cigarettes has been identified as one of the most critical risk factors contributing to COPD-related deaths [6, 7]. Numerous studies have confirmed and established a stable animal model of COPD by continuously exposing animals to cigarette smoke for more than 180 days [8], but recently combining lipopolysaccharides (LPS) for inducing inflammation and hypersecretion of mucus is becoming predominant [9]. It has been reported that cigarette smoke along with LPS leads to over-expression of proinflammatory cytokines such as interleukin (IL) 1β, tumor necrosis factor-α (TNF-α), IL-6 and interferon-γ (INF-γ). In COPD the inflammation of airways is mediated by neutrophils and not by eosinophils, which is evidenced by presence of a higher number of neutrophils in bronchoalveolar lavage (BAL) fluid [10]. Currently a number of synthetic agents such as steroids and NMDA inhibitors are used for treating COPD but the use of these agents is associated with adverse effects; hence alternate herbal agents are of utmost importance. Cigarette smoke mediated COPD is characterized by the presence of inflammation, vascular remodeling, smooth muscle hypertrophy and emphysema [11].
Rutin is a plant-derived flavonoid obtained primarily from citrus fruits [12]. Rutin shows a wide range of actions such as antioxidant, anticancer, anti-diabetic and anti-inflammatory properties [13]. In addition, rutin has significantly less toxicity compared to other bioflavonoids. Also it is potent as it is non-oxidizable and hence it is the safest among all the flavonoids. A previous report evidenced that when combined with vitamin C rutin demonstrated a protective effect in lung injury [14]. However, there are no reports indicating use of rutin in cigarette smoke induced COPD. Looking into the beneficial role of rutin in lung injury here we screened the anti-inflammatory effects of rutin on inflammation in lungs in a mouse model of cigarette smoke induced COPD and the mechanism involved.
Material and methods
Reagents and chemicals
Rutin hydrate HPLC grade was procured from Sigma Aldrich USA. It was solubilized in phosphate buffered saline (PBS). Tween-20, fetal bovine serum (FBS), and coating buffer were obtained from Thermo Fisher, USA. Roflumilast (RFL), an anti-inflammatory drug, was used as a positive control which was obtained as a gift sample from the pre-clinical department of Affiliated Wujiang Hospital of Nantong University China. RFL was dissolved in PBS. All the other reagents and chemicals used were of AR grade.
Animal groups, conditions and treatments
For the present study we used female BALB/c mice aged between 6 and 7 weeks; each group comprised 6 animals. The animals studies were approved by the institutional animal ethical review board of the Affiliated Wujiang Hospital of Nantong University, China; the approval number was NU147R. The animals were exposed to fresh or cigarette smoke air in a chamber supplied by Live cell Inst., South Korea. For the exposure protocol, all the animals were subjected to fresh air or cigarette smoke (6 units/day for 2 weeks); each cigarette smoke exposure lasted for 30 min with an interval of 5 min.
The treatment groups were group 1: control group (Fresh air exposed); group 2: cigarette smoke exposed group (CS group); group 3: RFL group (positive control group); group 4: CS + rutin (200 mg/kg); group 5: CS + rutin (300 mg/kg) and group 6: CS + rutin (400 mg/kg). The treatment with rutin lasted from the 5th day to the 13th day. On the 14th day (24 h after the last exposure) the animals were sacrificed and the lungs were collected; also the bronchoalveolar lavage fluid was collected for analysis. The experiments were repeated twice.
Analysis of bronchoalveolar lavage fluid
After the study protocol, 24 h after the last exposure, the animals were killed by cervical dislocation. For collecting the bronchoalveolar cells, the lungs of animals were flushed slowly with PBS using injection and the flushed fluid was collected by cannula from the tracheal portion. The cells collected in the fluid were counted using a hemocytometer; further for analyzing the differential cell counts, the fluid was spread on slides by the process of cytocentrifugation for 3 min at 300 rpm followed by staining (Diff-Quick). After this the bronchoalveolar fluid was centrifuged at 10000 rpm, and the obtained supernatant was frozen and stored at –80°C.
ELISA study of expression of TNF-α, PAF and IL-8 in bronchoalveolar lavage fluid
The total protein levels were estimated by a protein estimation kit (Thermo Fisher USA). The levels of TNF-α, IL-8 and platelet-activating factor (PAF) were measured by ELISA kit (Thermo Fisher USA). For the analysis a 96-well plate was incubated with anti-rat IL-8, PAF and TNF-α monoclonal antibodies for 12 h at 4°C in coating buffer. The plates were washed with 0.05% Tween-20 PBS solution and blocked with fetal bovine serum 5% for 1 h at 25°C. After this, the bronchoalveolar lavage fluid (100 µl) was stored at room temperature for 1 h. After this the anti-rat TNF-α, IL-8 and PAF monoclonal secondary antibodies were incubated in PBS with 5% FBS for 1 h. Finally, the plates were exposed with TMB solution for 20 min and the process was halted using a stop solution. The samples were measured for optical density in a microplate reader (Thermo Fisher USA) at 450 nm.
Histopathology of lung tissue
At the end of the study the animals were euthanized and lung tissues were harvested for histopathological analysis. The lower lobes of lung tissues were used for the study; the lungs were filled with paraformaldehyde (4%) solution. The lung tissues were then dehydrated and fixed in paraffin. The embedded tissues were sliced into sections of 4 µm on a rotary microtome; the sections were mounted on glass slides, deparaffinized and subjected to hematoxylin and eosin (H&E) staining. The tissue sections were analyzed for peri-bronchial inflammation as reported earlier [15]. The sections of tissue were stained using periodic acid Schiff (PAS) staining for analyzing the hyperplasia in the goblet cells. The PAS positive stained cells among the total epithelial cells and in the epithelium were recorded. For evaluating the air space in the lungs morphometric analysis was done; tissue sections having the maximum parenchyma cross sections were selected, analyzed and imaged using image scanning software as described earlier [16].
Statistical analysis
All the results are presented as mean ± %RSD. All the statistical analysis was done using GraphPad prism (version 5) software (USA). The significance of differences between the groups was determined by performing one-way ANOVA and the Newman-Keuls multiple comparison test. Values of p < 0.05 were regarded as significant.
Results
Rutin decreased the numbers of total cells and inflammatory cells in bronchoalveolar lavage fluid
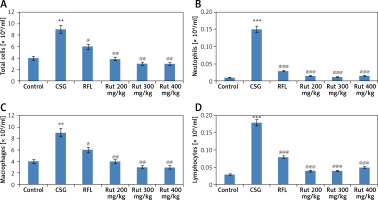
We assessed the anti-inflammatory effect of rutin at three different doses (200, 300 and 400 mg/kg of body weight) on cigarette smoke induced COPD. It was evidenced that rutin failed to show significant effect at 100 mg/kg dose, but a dose of 300 mg/kg showed a significant decrease in count of leukocytes, lymphocytes, neutrophils and macrophages in the bronchoalveolar lavage fluid against the cigarette smoke group (Table I). For establishing the optimum dose of rutin we performed a separate study at 3 different doses of rutin, i.e. 200 mg/kg, 300 mg/kg and 400 mg/kg of body weight. It was observed that the cigarette smoke treated group demonstrated an elevated number of total leukocytes, lymphocytes, macrophages and neutrophils compared with the animals exposed to fresh air (control group). It was observed that the treatment group rutin 200 mg/kg, 300 mg/kg and 400 mg/kg or RFL showed a significant decrease in number of total lymphocytes, macrophages, leukocytes and neutrophils in the bronchoalveolar lavage fluid compared to those exposed to cigarette smoke (Figure 1).
Table I
Analysis of cells in bronchoalveolar lavage fluid
| Cells [× 104/ml] | Control | Cigarette smoke exposed | Cigarette smoke + RFL | Cigarette smoke + rutin (200 mg/kg) | Cigarette smoke + rutin (300 mg/kg) | Cigarette smoke + rutin (400 mg/kg) |
|---|---|---|---|---|---|---|
| Neutrophils | 0.00 ±0.00 | 0.32 ±0.01*** | 0.09 ±0.001## | 0.29 ±0.01 | 0.16 ±0.002 | 0.05 ±0.001### |
| Macrophages | 2.76 ±0.55 | 12.22 ±1.02*** | 6.21 ±1.06## | 11.10 ±1.22 | 8.11 ±1.33 | 4.65 ±1.66### |
| Lymphocytes | 0.03 ±0.001*** | 0.15 ±0.01# | 0.08 ±0.001 | 0.14 ±0.003 | 0.11 ±0.005 | 0.04 ±0.001### |
| Total cells | 2.85 ±0.012 | 13.11 ±1.11*** | 6.59 ±0.66# | 12.04 ±1.01 | 8.59 ±0.88 | 5.15 ±0.54### |
Figure 1
Microscopic findings and cell profiles in bronchoalveolar lavage fluid present in the intrapulmonary bronchi. The animals were sacrificed followed by infusion of phosphate buffered saline in the lungs and collection through a cannula into the trachea. A – Count for total cell numbers, B – total neutrophil count, C – total count for macrophages, D – total count for lymphocytes. The results are mean ± SEM. The statistical significance was established by one-way ANOVA followed by Newman-Keuls multiple comparison analysis (***p < 0.001, **p < 0.01 compared to control group, ###p < 0.001. ##p < 0.01, #p < 0.05 compared to cigarette smoke exposed group)
Rutin suppressed the expression of TNF-α, IL-8 and PAF in bronchoalveolar lavage fluid of cigarette smoke exposed mice
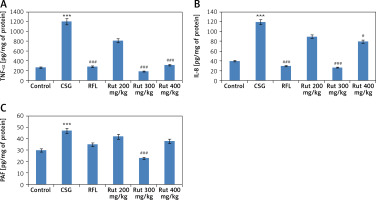
TNF-α, IL-8 and PAF are identified to be pro-inflammatory cytokines; all of them are involved in inflammatory disorders such as asthma [17]. PAF is reported to enhance the monocytes’ toxicity [18]. For studying the anti-inflammatory activity of rutin in lung tissues, the bronchoalveolar lavage fluid was collected and was evaluated for expression of these pro-inflammatory cytokines and chemokines. It was observed that the cigarette smoke exposed mice had significantly increased levels of IL-8, TNF-α and PAF in the bronchoalveolar lavage fluid compared to the animals exposed to fresh air. The positive control group receiving treatment with RFL exhibited decreased expression of TNF-α and IL-8 compared to negative control animals. However, the RFL-treated mice did not show a decrease in the levels of PAF. Rutin at doses of 200 and 300 mg/kg body weight demonstrated decreased TNF-α and IL-8 levels in the bronchoalveolar lavage fluid compared to negative control mice. However, suppression of levels of PAF was observed only in mice treated with 300 mg/kg dose compared to the negative control group; also the treatment showed similar effects on TNF-α, IL-8 and PAF levels in the control fresh air treated group. However, the dose of rutin 100 mg/kg did not show a significant effect on decreased levels of TNF-α, IL-8 and PAF compared to the negative control group (Figure 2).
Figure 2
Effect of treatment of rutin on levels of TNF-α, IL-8 and PAF in bronchoalveolar lavage fluid collected from experimental animals. A – Levels of TNF-α, B – levels of IL-8, C – Levels of PAF as estimated by ELISA. The results are mean ± SEM. The statistical significance was established by one-way ANOVA followed by Newman-Keuls multiple comparison analysis (***p < 0.001 compared to control, ###p < 0.001, #p < 0.05 compared to cigarette smoke exposed group)
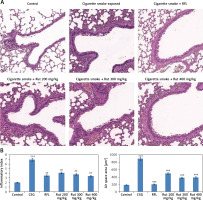
Rutin decreases alveolar damage in cigarette smoke exposed mice
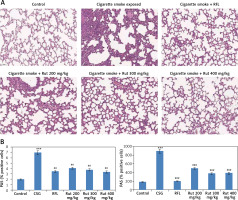
To evaluate whether rutin showed a protective effect on cigarette smoke mediated lung damage, the obtained lung tissue sections were subjected to hematoxylin and eosin staining (H&E). The outcomes of histopathology of lung tissues showed a similar pattern of cell numbers as observed in bronchoalveolar lavage fluid. There was a significant influx of inflammatory cells into the intraluminal areas as well as in peribronchial layers of the tissue sections of cigarette smoke exposed mice. Treatment with rutin caused a significant decrease in infiltration of inflammatory cells in the lungs of cigarette smoke exposed mice, which was similar to the RFL-treated mice. These findings suggested that treatment with rutin inhibited the cigarette smoke mediated inflammation in lung tissues similar to the positive control RFL mice (Figures 3 A, B). Also it was observed that the exposure to cigarette smoke in mice led to enlargement of the airspace. The results of quantitative analysis suggested that the cigarette smoke exposed group showed signs of alveolar damage, which caused an increase in air spaces suggesting an abnormal increase in size of air spaces compared to the rutin-treated group of mice. In contrast the group treated with rutin showed decreased alveolar damage (Figure 3 C).
Figure 3
Effect of treatment of rutin on morphological changes in cigarette smoke exposed animals. A – The rats were sacrificed and the right lower lobes of experimental mice were isolated and stained with H & E staining. B – The air space was recorded using Image-Pro plus 5.1 software. The results are mean ± SEM. The statistical significance was established by one-way ANOVA followed by Newman-Keuls multiple comparison analysis (***p < 0.001 compared to control, ##p < 0.01 compared to cigarette smoke exposed mice)
Treatment with rutin ameliorates cigarette smoke mediated lung inflammation
For assessing goblet cell hyperplasia, the tissue sections were stained with periodic acid-Schiff (PAS) (Figure 4 A). As reported earlier, the PAS staining positive goblet cells present in the periphery of the epithelium pathway of bronchi were observed primarily in the cigarette smoke exposed mice compared to the control group. However, treatment with rutin decreased the PAS positive cell number around the epithelium of bronchi (Figure 4 B). Cumulatively, the results suggested that rutin exerts a potentially beneficial effect on cigarette smoke mediated inflammation in lungs.
Figure 4
Effect of treatment of rutin on morphological changes in cigarette smoke exposed animals. A – The rats were sacrificed and the right lower lobes of experimental mice were isolated and stained with H & E staining. B – The air space was recorded using Image-Pro plus 5.1 software. The results are mean ± SEM. The statistical significance was established by one-way ANOVA followed by Newman-Keuls multiple comparison analysis (***p < 0.001 compared to control, ##p < 0.01 compared to cigarette smoke exposed mice)
Discussion
Inflammation is one of the main features of COPD; it is associated with release of various inflammatory mediators such as IL-8, leukotriene B4 and chemokine ligand 6, and these are chemoattractants for chemokines and neutrophils such as monocyte chemoattractant protein-1 and macrophage inflammatory proteins responsible for attracting macrophages [19]. Accumulation of neutrophils, dendritic cells and macrophages is seen in COPD [20]. Here, we observed infiltration of inflammatory cells in the bronchoalveolar lavage fluid and in parenchyma of lungs after cigarette smoke exposure. Neutrophils are responsible for inducing tissue damage in COPD by releasing various inflammatory mediators such as elastases and matrix metalloproteinase [21].
In the present study, due to exposure of cigarette smoke in experimental mice the influx of neutrophils resulted in elevated levels of TNF-α, IL-8 and PAF in the bronchoalveolar lavage. In addition to this, the exposure to cigarette smoke mediated hyperplasia of goblet cell is linked with induction of bronchitis, which is associated with COPD [22]. Hyperplasia of goblet cells is one of the morphological changes in the lung epithelium; also there are various epithelial alterations which include hypertrophy of submucosal glands along with loss of cells causing decreased mucociliary clearance and formation of mucus [23].
Exposure to cigarette smoke can lead to progression of disorders such as asthma, lung cancer, cardiovascular disorders and COPD. The cigarette smoke contains a number of compounds which include damaging reactive oxygen species which could damage DNA and could activate the PARP enzyme [24].
In the current research, the influx of inflammatory cells in the bronchoalveolar lavage fluid was seen after the exposure to cigarette smoke. Also, the cigarette smoke resulted in accumulation of inflammatory cells in the lung parenchyma, increased secretion of mucus, goblet cell hyperplasia and alveoli enlargement. Here, we observed changes which were very like those observed in patients with COPD [21]. In the study it was observed that after exposing the mice to cigarette smoke the treatment with rutin showed efficacy which was comparable to the positive control group treated with RFL, which is currently the commercially available anti-COPD agent [25].
Previously several studies involving molecules from herbal sources have been used in correcting COPD and related disorders. Radix Stemonae is a traditional Chinese medicine used as an anti-tussive agent; recently it was reported to show a protective effect in a COPD model in rats [26]. Asparagus cochinchinensis has been reported to exert a protective effect in asthma [27]. The extract of Schisandra chinensis has been evidenced to show an antitussive effect in cigarette smoke exposed guinea pigs [28]. Rutin is a molecule of the flavonol category; it has been reported to exert various activities such as anti-cancer, anti-inflammatory and anti-tussive. The identified molecular targets of rutin are TNF-α, IL-1β, PARP, iNOS, IL-6 and IL-8 [29]. So looking into the activities and targets of rutin it can be beneficial in correcting inflammatory disorders such as COPD. However, there are no reports exploring rutin in cigarette smoke induced animal models. This is the first time that rutin has been tested for its action in cigarette smoke exposed mice. In the present study rutin at three doses (200, 300 and 400 mg/kg body weight) decreased the influx of inflammatory cells in the bronchoalveolar lavage fluid significantly. Furthermore, when the histological characters were assessed in the lung tissues, rutin (300 mg/kg) suppressed the influx of neutrophils, lymphocytes and macrophages and the goblet cell metaplasia. In addition, the treatment with rutin also inhibited the thickening of epithelial basement membrane and inflammation of the bronchi. Rutin at a dose of 300 mg/kg also significantly decreased the IL-8, TNF-α and PAF levels compared to cigarette smoke exposed mice. However, rutin at the dose of 200 mg/kg failed to show a significant effect compared to the other two high doses of 300 and 400 mg/kg on the production of IL-8, TNF-α and PAF.
IL-8, also known as interlukin-8, is a known chemokine secreted by the macrophages, epithelial cells and airway smooth muscles [30]. TNF-α is a pro-inflammatory cytokine which is reported to activate other cytokines such as Il-8 and IL-6 [31]. It has also been found to be responsible for increasing the gene transcription of IL-8 [21]. Platelet-activating factor (PAF) is reported to be a potential lipid mediator implicated in disorders such as acute lung injury, asthma and ischemia/reperfusion injury [32].
In the present work, rutin suppressed the production of pro-inflammatory cytokine in cigarette smoke exposed mice in an acute as well as a chronic lung inflammatory condition. Hence we believe that rutin may steadily act on downstream effects which include infiltration of inflammatory cells and inflammatory mediators. Parallel to this, the histopathological data suggested that treatment with rutin halted the progression of airspace enlargement and also the hyperplasia of goblet cells. The findings showed a beneficial effect of rutin for structural changes of lung tissues in the cigarette smoke induced COPD condition in an animal model.
In conclusion, the findings of the present work provide concrete proof about the treatment with rutin showing beneficial effects against the cigarette smoke mediated inflammation in lungs of mice. The activity against the inflammatory mediators clearly confirms the potential of rutin in treating COPD. However, a detailed study elucidating the mechanism of action of rutin should be done.