Lipid disorders: an overview of the most preventable cardiovascular risk factor
Deaths worldwide are primarily related to cardiovascular diseases, among which atherosclerotic cardiovascular disease (ASCVD) plays a prominent role (20 million annually). In 2019, ASCVDs accounted for more than two-thirds of cardiovascular disease-related deaths overall (coronary artery disease [CAD] – 49.2%; ischaemic stroke – 17.7%; peripheral artery disease [PAD] – 0.4%) [1]. Invariably, for many years, the main risk factors for ASCVD, irrespective of gender, have included hypertension, poor dietary habits and lipid disorders (elevated low-density lipoprotein cholesterol [LDL-C]) [1–3]. At the American College of Cardiology (ACC) 2024 Congress in Atlanta, findings were presented indicating that more than 88% of CAD, the leading cause of death worldwide, is specifically related to the presence of inadequately controlled risk factors [4]. The global burden of CAD has steadily increased over the past three decades, in parallel with the escalation of risk factors [4].
The relationship between LDL-C concentration and ASCVD risk depends on the degree of increase in LDL-C concentration and the duration of exposure to this elevated concentration (called cholesterol-years) [2]. The theoretical cumulative threshold of LDL-C exposure, at which ACS is more likely to occur, has been set at approximately 8,000 mg/dl-years; such a threshold is reached at different ages depending on individual LDL-C levels and duration of exposure [5]. For example, the estimated age of onset of acute coronary syndrome (ACS) in a patient with LDL-C levels of 200 mg/dl, 125 mg/dl and 80 mg/dl is approximately 40, 60 and 100 years, respectively [5, 6]. Hence, in a patient with untreated heterozygous familial hypercholesterolaemia (HeFH) or lifestyle-related severe hypercholesterolaemia with a high baseline LDL-C, the mean age of onset of acute coronary syndrome (ACS) might be 40–45 and 55–60 years, respectively, and 70–75 years in the general population [5]. It is noteworthy that the risk of ACS depends on the cumulative exposure to LDL-C depending on the period in life when it started. The same cumulative exposure if it occurred in a younger person is associated with a higher risk of ACS than in an older person [7]. This is crucial because lipid disorders are an important factor in premature myocardial infarction (which currently accounts for 25% of all ACS cases), defined as women < 55 years, men < 60 years [8]. A 50% reduction in LDL-C levels reduces cardiovascular risk irrespective of the duration of hypercholesterolaemia, but to the greatest extent when achieved at the earliest possible stage of the disease (starting treatment at age 30 – hazard ratio [HR] = 0.48; 40 – HR = 0.54; 50 – HR = 0.63; 60 years – HR = 0.73) [9]. Each 1 mmol/l reduction in serum LDL-C is associated with a 12% (95% CI: 8–16%) reduction in the risk of major cardiovascular events (MACE) in year 1, 20% (16–24%) in year 3, 23% (18–27%) in year 5 and 29% (14–42%) in year 7 of lipid-lowering treatment [10]. It should be emphasised that intensive lipid-lowering treatment (LLT) leading to LDL-C reduction < 40 mg/dl (< 1 mmol/l) is safe and does not increase the risk of neurocognitive impairment, cancer, haemorrhagic stroke, type 2 diabetes, hepatobiliary disorders, muscular impairment or cataracts, while it allows an even greater reduction in the risk of cardiovascular events (OR = 0.82; 95% CI: 0.72–0.94) [11].
In primary prevention, LLT is associated with a reduction in the risk of death from any cause by 11%, death from cardiovascular causes by 20%, ACS by 38%, stroke by 17%, unstable ischaemic heart disease (IHD) by 25% and MACE by 26% [12]. In secondary prevention, LLT leads to a reduction in the risk of death from any cause by 22%, death from cardiovascular causes by 31%, ACS by 38%, the need for coronary revascularisation by 44% and cerebrovascular events by 25% [13]. The above information clearly shows that the treatment of lipid disorders should be carried out according to the principles continuously advocated for the last several years by, among others, the International Lipid Expert Panel (ILEP) and the Polish Lipid Association (PoLA): “the earlier the better”, “the lower the better” and “the longer the better” [14]. Only such an approach allows the maximum reduction of exposure to elevated LDL-C over the life course. It is noteworthy that lipid disorders are the most preventable risk factor for ASCVD, allowing risk reductions of up to 60% assuming early achievement of the therapeutic goal and maintenance throughout lifetime. In 1990, 3 million people died worldwide due to high LDL-C levels, and in 2019, 4.4 million people [15]. If, hypothetically, patients had a therapeutic target of LDL-C set, there would be a reduction of 4.4 million deaths per year worldwide and up to 30–40,000 only in Poland [16].
Rosuvastatin and ezetimibe SPC treatment – when and in whom?
The target level of lipid parameters depends on the individual cardiovascular risk of each patient (Tables I and II) [14, 17].
The 2019 Guidelines of the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) indicate that in high and very high cardiovascular risk patients, the target LDL-C level should be < 55 mg/dl (1.5 mmol/l; including a reduction in baseline level by ≥ 50%) and < 70 mg/dl (1.8 mmol/l; including a reduction in baseline concentration by ≥ 50%), respectively – Class I recommendations [18]. The pharmacological treatment recommendations correspond fully with this, as they indicate that a potent statin should be used at the highest tolerated dose to achieve the target LDL-C level (Class I recommendations). In high-risk and very high-risk patients, when monotherapy with a potent statin at the highest tolerated dose does not achieve the therapeutic goal, ezetimibe should be added (Class I recommendations) [18]. This means that the EAS/ESC 2019 guideline, as well as the subsequent ESC guidelines on prevention of cardiovascular disease (2021) [19], indicate step-by-step intensification of lipid-lowering treatment. The Polish Lipid Association (PoLA) 2021 guidelines elaborate the definition of patients with extreme cardiovascular risk and propose a more intensive treatment. The PoLA guidelines, in line with the recommendations of the International Lipid Expert Panel (ILEP) of April 2021 [20], indicate that in extreme (see extended definition in Table I) and very high-risk patients, in whom it is known in advance that monotherapy with a potent statin will not achieve the therapeutic goal (in most of the patients), immediate/upfront treatment with a combination of a potent statin at the maximum tolerated dose with ezetimibe should be applied [14, 20]. This is because each of these patient groups needs to reach therapeutic targets as early as possible, in order to minimise their cumulative lifetime exposure to elevated LDL-C levels [2]. The 2023 ESC guidelines for the management of ACS contain a slightly modified recommendation for lipid-lowering treatment. Drawing on, inter alia, the work of the Polish experts, it was recommended that upfront combination therapy with a potent statin + ezetimibe may be considered in ACS patients (recommendation class IIb) [21].
Table I
1 SCORE2 risk > 25% corresponds to e.g. woman aged 65 years, smoking, with a systolic blood pressure of 165 mm Hg and non-HDL cholesterol of 155 mg/dl (4 mmol/l) or man aged 60 years, smoking, with a systolic blood pressure of 165 mm Hg and non-HDL cholesterol of 194 mg/dl (5 mmol/l); estimated LDL-C > 160 mg/dl (4.1 mmol/l).
2 Polyvascular disease (= multilevel atherosclerosis) – the presence of significant atherosclerotic lesions in at least two of the three vascular beds – coronary vessels, carotid and vertebral arteries and/or peripheral vessels.
3 Organ damage is defined as the presence of microalbuminuria, retinopathy, neuropathy and/or left ventricular myocardial damage.
5 Major risk factors are age ≥ 65 years, hypertension, dyslipidaemia, smoking, obesity; not applicable to type 1 diabetes in young adults (< 35 years of age) with diabetes duration of < 10 years. When assessing renal function, it is recommended to determine albuminuria using the albumin/creatinine ratio (ACR).
The latest 2024 ILEP recommendations for lipid-lowering treatment in very high CVD risk patients have introduced significant modifications to treatment [22]. In patients with established ASCVD prior to any cardiovascular event (without ACS, HeFH, extreme cardiovascular risk, diabetes or statin intolerance), it is recommended to administer upfront lipid-lowering treatment with a combination of a potent statin at the maximum tolerated dose and ezetimibe, preferably in the form of a single pill combination (SPC). In patients at extreme cardiovascular risk, the ILEP 2024 guidelines advocate upfront lipid-lowering therapy with triple SPC including a potent statin at the maximum tolerated dose with ezetimibe and inhibitors/modulators of proprotein convertase subtilisin/kexin type 9 (PCSK9 inhibitors and inclisiran) or a statin with ezetimibe and bempedoic acid (obviously if available and possible based on the country-specific reimbursement criteria). The ILEP 2024 guidelines also include precise recommendations for the personalisation of LLT in patients with partial/complete statin intolerance or metabolic disorders [22]. This is new, as the ESC/EAS guidelines of 2019 [18] as well as the ESC ACS guidelines of 2023 [21] or the most recent guidelines for the management of chronic coronary syndromes (2024) [23] recommend a stepwise intensification of lipid-lowering treatment (starting with a statin, then adding ezetimibe if the target is not reached after 4–6 weeks and in the next step adding a PCSK9 inhibitor, inclisiran or bempedoic acid) [18, 21,23]. The approach recommended by ILEP 2024 is much closer to daily clinical practice and has much greater benefits for patients (faster achievement of the target, fewer treatment discontinuations and side effects, greater CVD risk reduction), as clearly demonstrated in clinical trials and their meta-analyses [24].
In a registry study involving 72,050 patients undergoing percutaneous coronary intervention (PCI), which assessed the effect of rosuvastatin monotherapy compared with rosuvastatin + ezetimibe combination therapy on the risk of occurrence of a composite endpoint (3-year composite event encompassing cardiovascular death, ACS, coronary revascularisation, hospitalisation for heart failure or non-fatal stroke), upfront combination LLT was found to be associated with a lower risk of the composite endpoint (HR = 0.75; 95% CI: 0.7–0.79), lower risk of treatment discontinuation (HR = 0.85; 95% CI: 0.78–0.94) and lower risk of new cases of diabetes (HR = 0.80; 95% CI: 0.72–0.88) [25]. Extremely important clinical guidance was provided by a study by Lewek et al. involving 38,023 ACS patients from the Polish Registry of Acute Coronary Syndromes (PL-ACS). The study showed that initial combination therapy (potent statin + ezetimibe) was associated with a significant reduction in all-cause mortality compared with strong statin monotherapy (OR = 0.53; 95%CI: 0.38–0.733, with an absolute risk reduction of 4.7% at 3 years (number needed to treat [NNT] = 21), and a significant effect was observed after only 52 days of treatment [26]. A study by Jang et al. involving 21,446 ACS patients also demonstrated a significant advantage of initial statin + ezetimibe combination treatment, finding that this approach reduced the risk of ACS, stroke and all-cause mortality by 15% (HR = 0.85; 95% CI: 0.78–0.92) compared with statin monotherapy [27]. A meta-analysis of six randomised clinical trials (RCTs) by Oliveira et al., involving 2,574 ACS patients, confirmed that combination treatment (statin + ezetimibe) has greater cardiovascular benefits: an additional 7% reduction in the risk of MACE (RR = 0.93; 95% CI: 0.90–0.97) and non-fatal ACS by 12% (RR = 0.88; 95% CI: 0.81–0.95) [28]. It is also worth mentioning that statin + ezetimibe combination therapy may have benefits in primary prevention (prevention of first MACE). A study by Jun et al. involving 69,488 participants showed that the use of statin + ezetimibe combination therapy compared with statin monotherapy in at-risk patients in primary prevention led to a 19% risk reduction: ACS (HR = 0.81; 95% CI: 0.71–0.94) and stroke by 22% (HR = 0.78; 95% CI: 0.65–0.93) [29].
A meta-analysis of 14 studies (11 randomised controlled trials and 3 cohort studies) with 108,353 very high-risk patients showed that combination LLT significantly more effectively reduced the LDL-C level from baseline (MD, –12.96 mg/dl, 95% CI: –17.27 to –8.65), and significantly reduced all-cause mortality (OR = 0.81, 95% CI: 0.67 to 0.97), MACE (OR = 0.82, 95% CI: 0.69 to 0.97), and stroke incidence (OR = 0.83 95% CI: 0.75 to 0.91), when compared with statin monotherapy. The risk of adverse events and the therapy discontinuation rate were comparable between groups, and even significantly reduced (discontinuation by 44% for moderate intensity statin and ezetimibe) in the accompanying network meta-analysis [24]. A meta-analysis of five studies by Damarpally et al. including 48,668 patients with ACS showed that potent statin monotherapy was associated with higher mortality compared to statin + ezetimibe combination treatment (RR = 1.32; 95% CI: 1.18–1.47) [30]. Another meta-analysis of 12 studies by Shaya et al. demonstrated that after 6 months of combined statin + ezetimibe therapy versus statin monotherapy, a significantly greater reduction in LDL-C was observed in patients with ASCVD (–21.86 mg/dl; 95% CI: –26.56 to –17.17) as well as in ACS patients (–19.19 mg/dl; 95% CI: –25.22 to –13.16) [31]. The use of statin + ezetimibe combination therapy also significantly increases the chance of achieving target LDL-C levels (by as much as 85%) [24, 32].
The more beneficial cardiovascular effects associated with statin + ezetimibe combination therapy are due to the synergistic (and not just addictive) anti-atherosclerotic effects of these medicines. Statins reduce LDL-C levels, have antioxidant, anti-inflammatory and anticoagulant effects and can dissolve cholesterol crystals. Ezetimibe, on the other hand, also lowers LDL-C level (although through a different mechanism of action), has an antioxidant effect, reduces sterols, inhibits vascular smooth muscle cell proliferation and causes cholesterol crystals to dissolve [33]. The results of the abovementioned studies and the recent ILEP 2024 recommendations [22] should put an end to the debate over the rationale for starting lipid-lowering therapy with a potent statin at the maximum tolerated dose in combination with ezetimibe in patients who are at least at very high cardiovascular risk. Such management significantly improves the prognosis of these patients and should be the gold standard of treatment [34]. Unfortunately, despite the clear evidence of the enormous cardiovascular benefits and safety of lipid-lowering treatment, data indicate that the achievement of the LDL-C therapeutic goal as well as the use of combination therapy in high- and very high-risk patients is low. The SANTORINI study, which enrolled 9,044 patients at high or very high cardiovascular risk (from 14 Western European countries), found that only 20.1% of these patients (24% in the high-risk group and 18.6% in the very high-risk group) achieved the therapeutic goal according to the 2019 ESC/EAS guidelines [18, 35]. During the extended follow-up of the SANTORINI trial, the use of monotherapy and combination therapy increased from 53.6 and 25.6% to 57.1 and 37.9%, respectively. The mean LDL-C level decreased from 2.4 to 2.0 mmol/l. Goal attainment according to the 2019 EAS guidelines [18] improved from 21.2 to 30.9%, largely driven by LLT use among those not on LLT at baseline. LDL-C goal attainment was greater with combination therapy compared with monotherapy at follow-up (39.4 vs. 25.5%) [36].
From a clinical point of view, it is important to specify which strong statin should be used to start the treatment. Potent statins include rosuvastatin, atorvastatin and pitavastatin (according to the International Atherosclerosis Society [IAS], while Polish guidelines classify pitavastatin as having moderate to intense potency) [37, 38]. A comparison of the lipid-lowering efficacy of different statins resulted in the following ranking of their power to reduce LDL-C: rosuvastatin > atorvastatin > pitavastatin > simvastatin > pravastatin > fluvastatin > lovastatin > placebo. Rosuvastatin ranked first in terms of its efficacy to lower LDL-C and apolipoprotein B (apoB) levels and its power to increase apolipoprotein A1 (apoA1) levels [39]. Interestingly, when looking at the anti-inflammatory properties (effect on hsCRP), the order is slightly different, with rosuvastatin having the strongest anti-inflammatory effect, followed by fluvastatin, pitavastatin, atorvastatin, pravastatin, simvastatin and lovastatin [40]. Rosuvastatin is, according to the U.S. Food and Drug Administration (FDA), the most effective statin (by 4–6%) in reducing LDL-C concentrations versus double-dose atorvastatin. The EAS/ESC, PoLA 2021, ESC 2024 and ILEP 2024 guidelines all agree that the statin of first choice is the one with the greatest lipid-lowering potency [14, 18,21, 22]. According to the PoLA 2021 guidelines, atorvastatin is preferred in patients with chronic kidney disease [14]. The ASTEROID trial showed that rosuvastatin administered for 24 months at a dose of 40 mg led to regression of atherosclerotic coronary artery lesions (10.16 mm2 vs. 5.81 mm2) [41]. The SATURN study compared the effects of the highest doses of rosuvastatin and atorvastatin on atherosclerotic lesion progression. It showed that rosuvastatin had a higher lipid-lowering potency, while the effect on plaque volume reduction was similar for both statins tested [42]. Nevertheless, a meta-analysis of six RCTs by Kumar et al. showed that rosuvastatin reduced plaque volume and LDL-C concentration to a greater extent than atorvastatin [43]. Some risk of new cases of diabetes (NOD) is associated with the use of potent statins [44]. This risk can be optimised using statin combination therapy with ezetimibe (diminished risk of diabetes with improving LDL-C and outcomes reduction). The beneficial effect was demonstrated in the RACING trial, in which patients after PCI were given rosuvastatin (20 mg) in monotherapy versus rosuvastatin (10 mg) with ezetimibe. After 3 years, it was found that combination therapy was associated with a lower incidence of new cases of diabetes requiring pharmacotherapy (7.7% vs. 9.6%; HR = 0.80; 95% CI: 0.72–0.88). Furthermore, combination therapy was found to be better tolerated by patients, as discontinuation of treatment was observed significantly less frequently (6.5% vs. 7.6%; HR = 0.85; 95% CI: 0.78–0.94) [45]. Combination therapy with a statin and ezetimibe compared with monotherapy is significantly less likely to cause myalgia (RR = 0.27; 95% CI: 0.13–0.57), which means better therapy adherence and a significantly lower risk of treatment discontinuation related to adverse effects (RR = 0.61; 95% CI: 0.51–0.74) [46, 47]. It is worth pointing out that when statin substitution is needed (in patients with statin-associated muscle symptoms), rosuvastatin can be switched to atorvastatin without significant loss of cardiovascular protection [48]. The above observation was important in the development of the new ILEP 2024 recommendations, in which for patients with metabolic disorders (including diabetes) and patients with statin intolerance it is recommended to start treatment with a lower (not maximum) dose of statin (e.g. rosuvastatin 20 mg instead of 40 mg) along with ezetimibe, allowing the achievement of the therapeutic goal with fewer side effects and discontinuations and better adherence; this approach also may improve treatment of patients with suboptimal lipid-lowering therapy [22].
The guidelines rightly indicate that combination LLT should be based on SPCs as much as possible. It was demonstrated beyond any doubt that patients taking a statin with ezetimibe as an SPC, compared to those taking both medicines in the same doses in separate pills, had an 87% (RR = 1.87; 95% CI: 1.75–1.99) higher chance of good adherence. And, in turn, patients with high adherence had a 55% (RR = 0.45; 95% CI: 0.25–0.80) lower risk of cardiovascular events (cardiovascular mortality or cardiovascular hospitalisation) [49].
The benefits of upfront SPC lipid-lowering treatment with a potent statin at the maximum tolerated dose and ezetimibe in patients with ACS are summarised in Figure 1, while recommendations for the use of SPC (in this case rosuvastatin + ezetimibe) are presented in Table III.
Figure 1
Benefits of up-front administration of SPC with a potent statin at the maximum tolerated dose and ezetimibe in a patient with acute coronary syndrome. Study authors based the figure on information from multiple sources [22, 24,25, 47, 49]
ILEP – International Lipid Expert Panel, ACS – acute coronary syndrome, LDL-C – low-density lipoprotein cholesterol, CV – cardiovascular, MACE – major cardiovascular adverse event.
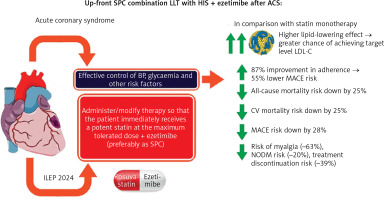
Table II
Table III
Recommendations for the use of rosuvastatin and ezetimibe SPC
Combination treatment with SPC of rosuvastatin and acetylsalicylic acid – when and for whom?
The use of acetylsalicylic acid (ASA) in cardiovascular prevention, especially primary prevention (prior to ASCVD), is still widely debated [50]. In the course of ASCVD and diabetes, impaired vascular endothelial function, excessive platelet reactivity and unfavourable atheroma remodelling resulting in a more atherothrombogenic environment are observed [51]. According to the 2021 ESC guidelines for cardiovascular prevention, ASA 75–100 mg/day is recommended for secondary prevention (recommendation class IA). For primary prevention, the ESC 2021 guidelines state that low-dose ASA may be considered for primary prevention in patients with diabetes and a high or very high risk of ASCVD, if there are no clear contraindications (recommendation class IIb A) [19]. It is recommended that a proton pump inhibitor (PPI) should be considered in patients receiving ASA and at increased risk of gastrointestinal bleeding (recommendation class IA) [19]. Antiplatelet therapy is not recommended in patients with low/moderate cardiovascular risk due to an increased risk of major bleeding (unfavourable balance of benefits and risks) (recommendation class IIIA) [19]. Selected ESC recommendations for the use of ASA in the treatment of certain diseases are summarised in Table IV [19, 21,23, 52, 53].
Table IV
Selected recommendations of the European Society of Cardiology (ESC) on the use of acetylsalicylic acid in various diseases [21, 23, 52–54].
[i] ASA – acetylsalicylic acid, ASCVD – atherosclerotic cardiovascular disease, CABG – coronary artery bypass grafting, PAD – peripheral artery disease, MACE – major adverse cardiovascular event, CCS – chronic coronary syndrome, PCI – percutaneous coronary intervention, DAPT – dual antiplatelet therapy.
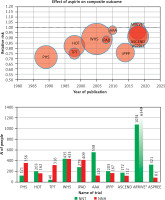
In primary prevention, the ESC 2021 guidelines [19] (and earlier US guidelines [54]) state that ASA use may be considered, after weighing potential benefits and risks, in patients with diabetes and high/very high cardiovascular risk. This recommendation is based on studies that show inconclusive evidence of significant benefits of using aspirin in primary prevention (Figure 2). Due to this inconclusive evidence on the ASA benefits in primary prevention, it is important to identify more specific patients at risk who might really benefit from this therapy. Available studies suggest that coronary computed tomography angiography (CCTA) with the calcium score (CS) may also be helpful in decision making, indicating that for a CAC-Score (coronary artery calcium score) > 100 there are significant benefits of using aspirin in the primary prevention of cardiovascular incidents, which has been confirmed in many studies [55]. A study by Ajufo et al. involving 2,191 participants in primary prevention showed that a higher CAC score was associated with an increased risk of bleeding and more clearly with ASCVD risk [56]. Compared with participants from the lowest CAC score group, those in the group with the highest CAC had an unadjusted hazard ratio (HR) of 2.6 (95% CI: 1.5–4.3; p < 0.001) for bleeding events and 5.3 (95% CI: 3.6–7.9; p < 0.001) for ASCVD events. After multivariable adjustment for risk factors, the association between CAC and bleeding became weaker (CAC ≥ 100 vs. CAC = 0; HR = 1.5, 95% CI: 0.8–2.6; p = 0.19), but the association between CAC and ASCVD remained significant in all CAC categories considered. There were no differences in the association of CAC with bleeding or ASCVD according to age above and below 50 years, black and non-black race, and male vs. female gender. Based on the obtained results, the authors concluded that people with CAC-Score > 100 and low bleeding risk can achieve significant cardiovascular benefits from taking ASA [56].
Figure 2
Summary of studies on the effect of aspirin use in primary prevention and the ratio of benefits (number needed to treat – NNT) to risks of adverse effects (number needed to harm – NNH). Based on: Della Bona R, Giubilato S, Palmieri M. et al. J Clin Med 2024; 13: 4148. https://doi.org/10.3390/jcm13144148 (reprint permission not required: CC BY licence) [50]
Thus, it is recommended that ASA in primary prevention should be considered in particular in patients with CAC ≥ 100, an increased risk of ASCVD (≥ 5%) and aged < 70 years [55].
It is worth citing here the 2019 cardiovascular prevention guidelines developed by the American College of Cardiology and the American Heart Association (ACC/AHA), recommending that low-dose ASA (7–100 mg orally daily) may be considered for primary prevention of ASCVD among selected adults aged 40 to 70 years who are at higher ASCVD risk but not at increased bleeding risk (recommendation class IIb A) [54]. At the same time, ACC/AHA 2019 advocates that low-dose aspirin (75–100 mg orally daily) should not be administered on a routine basis for primary prevention of ASCVD in adults > 70 years of age and adults of any age at an increased risk of bleeding (class III recommendation) [54].
In patients with diabetes, the use of ASA in primary prevention was associated with a significant 8% reduction in the risk of MACE, with a concomitant overall increase in the risk of major bleeding by 30% and the risk of major gastrointestinal bleeding by 39% [57]. The randomised ASCEND trial involving 15,480 patients with diabetes who received ASA vs. placebo for 7.4 years showed a significant 12% reduction in the risk of MACE (RR = 0.88; 95% CI: 0.79–0.97). The risk of major bleeding went up by 29% (RR = 1.29; 95% CI: 1.09–1.52) [58]. A 9% reduction in the risk of MACE (OR = 0.91; 95% CI: 0.84–0.99) was also found in a meta-analysis of eight studies by Ma et al., including 32,024 patients with diabetes [59]. A meta-analysis of 10 randomised clinical trials by Wang et al. showed that ASA use was associated with a significant reduction in the risk of MACE (RR = 0.89; 95% CI: 0.84–0.93), ACS (RR = 0.86; 95% CI: 0.78–0.95) and ischaemic stroke (RR = 0.84; 95% CI: 0.76–0.93); however, ASA also increased the risk of adverse events, i.e. major bleeding (RR = 1.42; 95% CI: 1.26–1.60), intracranial haemorrhage (RR = 1.33; 95% CI: 1.11–1.59) and gastrointestinal bleeding (RR = 1.91; 95% CI: 1.44–2.54). A more pronounced risk reduction in primary prevention was found in high-risk patients, those with diabetes and those aged ≤ 70 years [60]. The use of ASA in primary prevention may provide cardiovascular benefit only in patients with at least high cardiovascular risk (12% reduction in MACE [OR = 0.88; 95% CI: 0.80–0.97]) [61].
The use of a two-drug SPC containing rosuvastatin and ASA in patients for whom such treatment is indicated significantly improves cardiovascular prognosis. A study by Liu et al. including 3,778 individuals from the NHANES 2011–2018 database showed that rosuvastatin with ASA versus ASA monotherapy led to a 56% reduction in the risk of ASCVD (OR = 0.34; 95% CI: 0.23–0.50) in primary prevention [62]. It is worth noting that in elderly patients without ASCVD to whom ASA was administered despite a clear indication for such treatment, its discontinuation remains safe and does not increase cardiovascular risk in the short or long term [63].
Lp(a) – an additional argument for the use of aspirin in primary prevention?
The cardiovascular benefits observed as a result of ASA use in patients with diabetes are due to the residual risk of ASCVD [64]. Residual ASCVD risk consists of suboptimal LDL-C reduction, low-grade inflammation, prothrombotic factors, elevated triglycerides, elevated lipoprotein (a) (Lp(a)) and inadequate glycaemic control [64, 65]. ASA contributes to the optimisation of ASCVD risk through its effects on prothrombotic factors and, as recently shown, also on Lp(a). Studies to date on the use of aspirin in the context of prothrombotic risk associated with elevated Lp(a) concentrations have been inconclusive. However, the existence of such properties is beyond doubt, as the Lp(a) molecule is similar in structure to plasminogen. This is confirmed, for example, by the LIP(a)R registry, which demonstrated an independent association between increased Lp(a) levels (> 30 mg/dl/75 nmol/l) and platelet counts in patients at very high cardiovascular risk in secondary prevention [66].
Hence, a new potential indication to consider ASA for primary prevention is an elevated lipoprotein (a) [Lp(a)] level. A recent analysis of the Multi-Ethnic Study of Atherosclerosis (MESA) by Bhatia et al. involving 2,183 patients without a history of ASCVD showed that ASA use was associated with a significant 46% reduction in ASCVD risk among patients with elevated Lp(a) levels > 50 mg/dl (125 nmol/l) (HR = 0.54; 95% CI: 0.32–0.94). Those with Lp(a) levels > 50 mg/dl who used ASA had a similar ASCVD risk to those with Lp(a) levels ≤ 50 mg/dl, regardless of aspirin use [67]. Similar data were provided by a study by Razavi et al. involving 2,990 individuals from the National Health and Nutrition Examination Survey (NHANES III, 1988–1994) without a history of ASCVD. They found that regular ASA use was associated with a 52% lower risk of ASCVD mortality in those with Lp(a) levels > 50 mg/dl (HR = 0.48; 95% CI: 0.28–0.83), but not in those without elevated Lp(a) levels (HR = 1.01, 95% CI: 0.81–1.25) [68]. The results of this study are highly relevant because up to one in every 4-5 patients in Poland has an elevated Lp(a) level, i.e. > 6 million people [69]. It has been shown that among patients treated in Polish cardiology outpatient clinics, the prevalence of elevated Lp(a) levels is about 22%, and among patients with hyperlipidaemia it is 28% [70]. Notably, Lp(a) is an ASCVD risk factor independent of LDL-C (5-fold more atherogenic), whose concentration is mainly genetically determined [71, 72]. The launch of medicines targeting Lp(a) – pelacarsen, olpasiran, zerlasiran, lepodisiran and muvalaplin – is expected [72, 73]. Considering the use of ASA in patients at least at high cardiovascular risk in primary prevention with elevated Lp(a) levels may help to optimise the ASCVD risk associated with it.
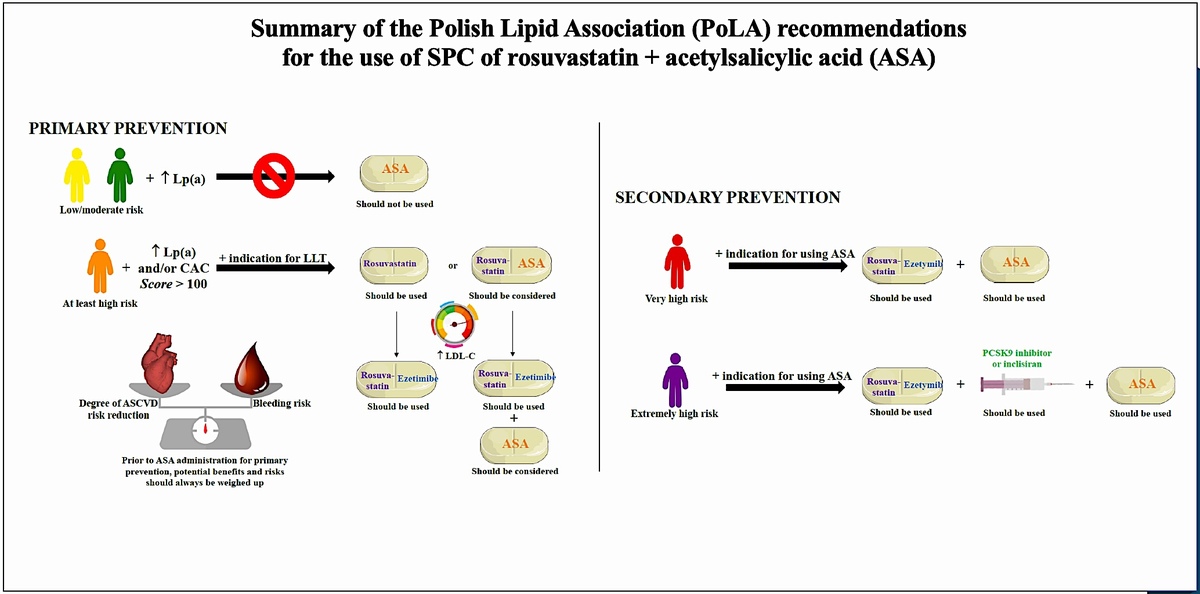
Table V and Figure 3 summarise the proposed recommendations for use of the two-drug SPC of rosuvastatin + ASA in cardiovascular prevention [74].
Figure 3
Summary of the Polish Lipid Association (PoLA) recommendations for the use of SPC of rosuvastatin + ASA. Definitions: Should be used (Class I), Should be considered (IIa).
ASCVD – atherosclerotic cardiovascular disease, Lp(a) – lipoprotein(a), ASA – acetylsalicylic acid, ASCVD – atherosclerotic cardiovascular disease, CAC – coronary artery calcium, LLT – lipid-lowering therapy, LDL-C – low-density lipoprotein cholesterol, PCSK9 – proprotein convertase subtilisin-kexin type 9, SPC – single pill combination, PPI – proton-pump inhibitor, ESC – European Society of Cardiology.

Table V
Summary of recommendations for the use of the rosuvastatin + acetylsalicylic acid (ASA) combination in patients for primary and secondary prevention
| Expert opinion |
|---|
PRIMARY PREVENTION
|
SECONDARY PREVENTION
|
| GENERAL |
ASA – acetylsalicylic acid, SPC – single pill combination, LDL-C – low-density lipoprotein cholesterol, PCSK9 – proprotein convertase subtilisin-kexin type 9.
* High risk of bleeding should be defined as: history of gastrointestinal bleeding or active peptic ulcer disease within the last 6 months, active liver disease (such as cirrhosis or active hepatitis) or history of aspirin intolerance; caution should also be exercised in the case of: malignancy diagnosed in the last 12 months, history of haemorrhagic or ischaemic stroke in the last 6 months, haemoglobin < 11 g/dl, thrombocytopenia < 100,000/µl and severe chronic kidney disease (GFR < 30 ml/min) [52, 74].
Summary and conclusions
Combination drugs, preferably as SPC, that improve adherence to treatment and achievement of therapeutic goals and help to reduce cardiovascular incidents and mortality are undoubtedly a way to improve poor treatment outcomes in Poland, where cardiovascular disease is responsible for up to 200,000 deaths per year, with the number of myocardial infarctions and strokes reaching 80,000 annually, 25% of people dying within 3 years after myocardial infarction and, finally, only 20–25% of patients at high or very high cardiovascular risk achieving the therapeutic target for LDL cholesterol. This expert opinion paper is intended to serve as practical guidance to optimise treatment for patients who have indications for lipid-lowering and antiplatelet therapy (up to several million people in Poland) using available rosuvastatin-based combination drugs. In this context, it should be emphasised that it is not possible to establish clear recommendations for the use of these combination therapies in individual patient groups, as data are still lacking (especially for combinations with aspirin) or are ambiguous in many cases, and in other cases there is an overlap of indications, which also points to the important role of treatment personalisation for patients at high and very high cardiovascular risk.