Introduction
Atrial fibrillation (AF) is the most common clinical arrhythmia, and AF-associated stroke is one of the most fatal complications [1], with a 5.6-fold higher risk of stroke than that in non-AF patients [2]. The standard therapy to prevent stroke is vitamin K antagonists (VKAs) or new oral anticoagulants (NOACs). However, the long-term use of oral anticoagulation (OAC) has the disadvantages of high bleeding risk, poor patient compliance, and heavy economic burden. Emerging evidence has showed that some patients with high risk of bleeding are unsuitable for this therapy. Recently, left atrial appendage occlusion (LAAO) has become an alternative strategy for stroke prevention in AF patients. LAAO is performed with a specially designed occluder to close the LAA, which is the source of 90% of AF emboli [3]. Many studies have shown that LAAO is a feasible and safe method and is associated with stroke risk reduction [4–7].
Animal studies have suggested that complete device endothelialization (CDE) is achieved at 45–90 days after the LAAO procedure, and the duration of postoperative antithrombotic therapy in humans is mainly based on these animal studies [8]. However, incomplete device endothelialization (IDE) at 3 months or later after LAAO was frequently reported in humans, which might result in device-related thrombus (DRT) formation and subsequent thromboembolic clinical events [9–12].
Thus, early detection of IDE is necessary to prevent DRT formation. However, there are few studies on the risk of IDE. In the present study, we aimed to investigate the risk factors and clinical impact of IDE following LAAO and provide a nomogram model for predicting the risks of IDE in AF patients after LAAO.
Material and methods
Study population and design
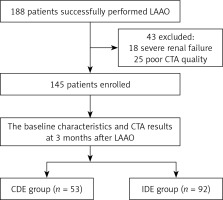
A total of 188 consecutive patients with AF undergoing successful LAAO between January 2019 and April 2022 at the Affiliated Taizhou People’s Hospital of Nanjing Medical University were enrolled. The diagnosis of AF was mainly based on the criteria listed in the 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with EACTS [13]. 43 patients were excluded because of unsuitability for dual source computed tomography angiography (CTA). Thus, the remaining 145 patients who had a complete 3-month clinical follow-up with CTA after LAAO were chosen for the final analysis (Figure 1): 78 patients with paroxysmal AF and 67 patients with persistent AF, aged from 36 to 85 years, with a mean age of 68.12 ±8.45 years. Based on the quantitative results of CTA, patients were divided into two groups: the IDE group and the CDE group. IDE and CDE were detected in 92 (63.4%) and 53 (36.6%) patients, respectively (Table I).
Table I
Comparison of relevant data between CDE group and IDE group
[i] CDE – complete endothelialization, IDE – incomplete device endothelialization, LAAO – left atrial appendage occlusion, CHD – coronary heart disease, M/H – monocyte/high-density lipoprotein, MR – mitral regurgitation, HDL – high-density lipoprotein, ALT – alanine aminotransferase, AST – aspartate aminotransferase, LA diameter – left atrial diameter, LVEF – left ventricular ejection fraction, LAA diameter – left atrial appendage diameter.
Patients were included if they met the following criteria: (I) aged 18 years or older with AF; (II) CHA2DS2-VASc score ≥ 2 (male) or ≥ 3 (female); (III) could not tolerate anticoagulation for a long period or had contraindications to anticoagulation; and (IV) had indications for LAAO, which was successfully performed using a Watchman 2.5 occluder device.
Patients were excluded if they presented with the following: (I) patients with moderate/severe mitral stenosis and those with a mechanical prosthetic heart valve(s); (II) left atrial diameter > 65 mm; (III) atrial thrombus detected by preoperative transesophageal echocardiographic (TEE) or CTA; (IV) a history of stroke within 1 month; (V) pericardial effusion or massive hemorrhage during surgery; or (VI) LAAO was performed with an occluder device other than the Watchman 2.5 occluder device.
LAAO procedure
As we described previously [14], patients underwent LAAO with TEE monitoring. A special sheathing canal was placed to perform left atrial appendage (LAA) angiography, and a pigtail angiographic catheter was directed to the LAA with the following positions: right anterior oblique (RAO) 30° + cranial (CRA) 20° and RAO30° + caudal (CAU) 20°. A suitable Watchman occluder was selected following measurement of LAA orifice width and depth. The Watchman occluder was introduced into the LAA along the sheathing canal. The position of the occluder was monitored by TEE. A pull test was conducted to determine the stability of the occluder. When a suitable position, stability and lack of trans-fabric leak > 5 mm were confirmed, the occluder was released.
Medical treatment
Since there were no clear guidelines about the anticoagulation or antiplatelet therapy after LAAO, as we described before [15], all patients in this study were treated with NOAC combined with antiplatelet medications (NOAC + SAPT therapy) for 3 months after LAAO. CTA was performed at 3 months after LAAO. If there was no DRT and the trans-fabric leak was < 5 mm, aspirin combined with clopidogrel was used for another 3 months, followed by aspirin or clopidogrel for life-long treatment.
CTA examination
Three months after LAAO, the Siemens SomatomForce 128-layer dual source CTA was used for scanning with a forward-looking ECG trigger sequence, automatic real-time control of radiation dose, and 30–80% R-R interval exposure. The contrast agent iodoprolamine (370 mgI/ml) was injected in a volume of 60–80 ml at a flow rate of 5.0 ml/s with a double-barrel high-pressure syringe, and then enhanced scanning was performed, ranging from 1 cm below the tracheal carina to the diaphragmatic surface of the heart. CT multiplanar reconstruction (MPR) technology was used to evaluate whether the occluder was completely endothelized.
Definitions
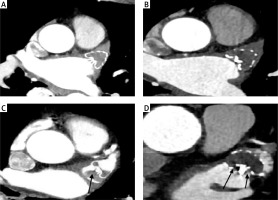
In this study CDE was defined as LAA attenuation < 100 HU or LAA/left atrium (LA) attenuation ratio < 0.25 (Figures 2 A, B). IDE was defined as LAA attenuation > 100 HU or LAA /LA attenuation ratio ≥ 0.25 and presence of trans-fabric leak CTA at 3 months after the procedure (Figures 2 C, D) [16, 17]. DRT was defined as nodular or mass-like enhancement defect with high grade hypo-attenuated thickening on the atrial aspect of the LAAO device images (Figure 2 C) [18, 19]. Trans-fabric leak was defined as contrast entering the LAA through the fabric rather than around the device (Figure 2 D).
Figure 2
Device endothelialization assessed by CTA. A, B – CDE of the Watchman device, no trans-fabric leak with LAA HU < 100 and LAA/LA < 0.25. C – IDE of the Watchman device, LAA attenuation > 100 HU or LAA/LA attenuation ratio – 0.25 with a DRT thrombus attached to the occluder device on the left atrial side. D – IDE of the Watchman device, LAA attenuation > 100 HU or LAA /LA attenuation ratio – 0.25 with a trans-fabric leak
Statistical analysis
SPSS 26.0 statistical software was used for data analysis. Continuous variables conforming to the normal distribution are presented as the mean ± standard deviation, while continuous variables not conforming to the normal distribution are presented as the median. Categorical variables are reported as counts and percentages. Normally distributed variables were compared using the independent t-test, while the z-test was used for non-normally distributed variables. The χ2 re test was used to compare between the two subgroups. Multivariate logistic regression analysis and collinearity analysis were used to evaluate risk factors for IDE. The receiver operating characteristic (ROC) curve was used to evaluate the value of risk factors in predicting IDE. The nomogram method was used to construct the risk prediction model of IDE after LAAO using R4.0.3 statistical software. The training set was resampled 1000 times for internal validation using the enhanced bootstrap method. The validation set data was externally validated and the discriminability of the model was evaluated using the index of concordance (C-index). A C-index of 0.5 indicated that the model had no predictive effect. The calibration curve was used to evaluate the consistency of the model. A value of p < 0.05 was considered as statistically significant.
Results
Comparison of baseline characteristics between CDE group and IDE group
LAAO with the Watchman 2.5 occluder was performed successfully in all patients. Meanwhile, all 145 patients completed the follow-up at 3 months after LAAO. There were 53 (34.6%) cases of CDE (CDE group) and 92 (63.4%) patients with IDE (IDE group). DRTs were found in 7 (4.8%) patients, in whom IDE occurred in 6 patients. Baseline characteristics are presented in Table I. Between the IDE group and the CDE group, there was no difference in sex, age, and type of atrial fibrillation (p > 0.05). However, there were significant differences between the two groups in serum urea, mitral regurgitation (MR, the degree is moderate to severe evaluated by transthoracic echocardiography), LAA diameter and occluder size (p < 0.05).
Multivariate logistic regression analysis of IDE after LAAO
As shown in Table II, multivariate logistic regression analysis indicated that serum urea, MR, LAA diameter, and occluder size were independent risk factors for IDE after LAAO. The regression coefficient (r), odds ratio (OR), and 95% confidence interval (CI) of serum urea were, respectively: –0.199, 0.819, and (0.679, 0.989); –1.406, 0.245, and (0.089, 0.676) for LAA diameter; –1.388, 0.250, and (0.092, 0.675) for occluder size; and –0.719, 0.487, and (0.244,0.972) for MR.
Table II
Logistic regression analysis of risk factors affecting IDE after LAAO
ROC curve analysis of independent risk factors of IDE after LAAO
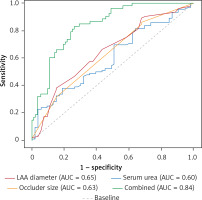
The AUC of serum urea for predicting IDE was 60.2% and the cut-off value was 7.05 mmol/l with a sensitivity of 38.0% and specificity of 81.1%. The AUC of LAA diameter for predicting IDE was 64.9%, with a sensitivity of 38% and specificity of 84.9%. The AUC of occluder size for predicting IDE was 63.2%, with a sensitivity of 85.9% and specificity 32.1%. The area under the AUC curve of the three factors combined to predict the IDE after LAAO was 0.84; the sensitivity and specificity were 83% and 74%, respectively (Figure 3).
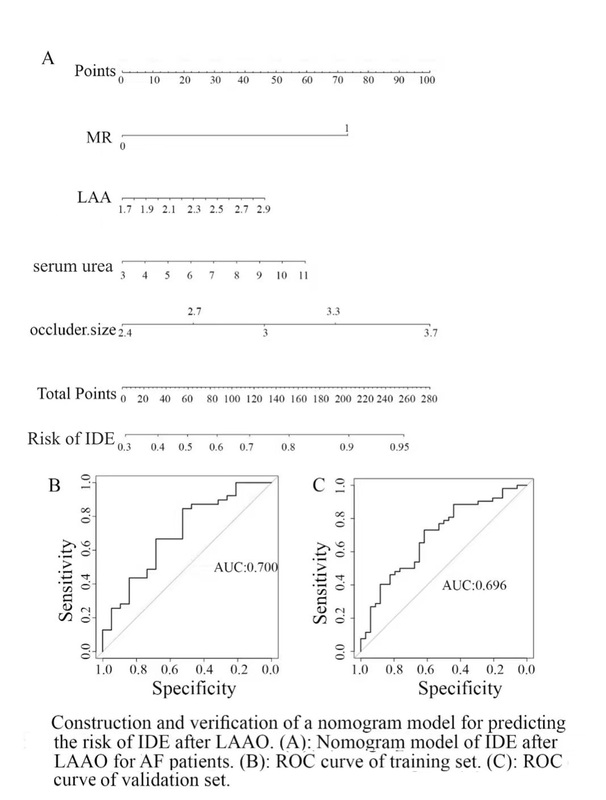
Construction and verification of a nomogram model for predicting the risk of IDE after LAAO
Accordingly, a nomogram model based on MR, LAA diameter, occluder size, and serum urea was constructed to predict the risk of IDE 3 months after LAAO in AF patients. As shown in Figure 4 A, with the extension of LAA diameter and occluder size, increase of serum urea, and combination with MR, the nomogram scores gradually increased and the risk degree increased accordingly. In the nomogram model, the scores of LAA diameter, occluder size, combined with MR and serum urea were 45, 97.5, 72.5, and 60, respectively. The total score obtained by calculating all risk factors could be used to estimate the risk of IDE after LAAO in patients with multiple risk factors, with a C-index of 0.70 (95% CI: 0.552–0.849), while the C-index of verification was 0.708 (95% CI: 0.596–0.820) (Figure 4 B), which indicated that the model had good accuracy and differentiation (Figure 4 C). Furthermore, multicollinearity diagnosis of LAA diameter, occluder size and serum urea was performed. The variance inflation factor (VIF) value of LAA diameter, occluder size and serum urea was 1.055, 1.051 and 1.009, respectively, which indicated that they were independent influencing factors.
Clinical outcomes at 3-month follow-up
During the 3-month clinical follow-up, no death, peripheral vascular embolism or transient ischemic attack occurred. Device-related thrombus (DRT) was observed in 5 cases, with 4 cases in the IDE group and 1 case in the CDE group. There were 2 patients who required hospitalization due to heart failure in the CDE group and 4 patients in the IDE group. Acute ischemic stroke was observed in 1 case in the IDE group. No statistically significant difference in clinical outcomes was observed in the present study between the IDE group and CDE group. The detailed results are shown in Table III.
Table III
Clinical outcomes at 3-month follow-up after LAAO
Discussion
The results of this study showed that MR, higher serum urea level, LAA diameter and large occluder size were associated with higher risk of IDE after successful LAAO. DRTs were found in 5 (3.45%) patients who received a Watchman device – 4 (4.34%) in the IDE group and 1 (1.89%) in the CDE group – which suggested that IDE might increase the incidence rate of DRT, although there was no significant difference in the DRT rate between the two groups at 3-month follow-up.
Experiments in animals have shown that the endothelialization process of the occluder is similar to trauma healing, which is a complex biological process of tissue repair [18]. However, the exact timing of the CDE after the LAAO remains uncertain. In canine models, full endothelialization occurred at 3 months [20]. The endothelialization process is roughly divided into three stages [8, 21,22].
Nevertheless, IDE of devices often occurred after LAAO in humans [23], with different occurrence rates. The reasons for this are related to heterogeneous definitions of IDE, various types of LAA occluder, and different cardiac imaging techniques. Recently, CTA appears to be a feasible alternative to TEE for post-LAA device surveillance to evaluate for DRT and peri-device leak (PDL) [16]. In our study IDE was defined as LAA attenuation > 100 HU or LAA/LA attenuation ratio ≥ 0.25 and presence of trans-fabric leak on CTA at 3 months post-procedurally, irrespective of PDL [16, 17]. This may partly account for the higher prevalence of IDE in our study. Similar results have been obtained by other researchers [24].
The potential factors of IDE after LAAO are still unclear. Meanwhile, it is important to investigate the risk factors for IDE, which would be helpful for better management of LAAO patients. However, except for a few case reports, there has been no systematic research focusing on endothelialization after LAAO in humans.
The results of this study showed that MR was an independent risk factor for IDE, which is consistent with Xu et al. [25], who reported a 70-year-old female patient with a diagnosis of persistent atrial fibrillation and moderate to severe MR who was found to have IDE of the occluder surface during transthoracic mitral valve replacement 1.5 years after LAAO. The reason may be due to the blood turbulence caused by moderate and severe MR, which has a long-term impact on the surface endothelialization of the occluder.
Our study also found that patients with a large LAA diameter and/or a large occluder in LAAO were most likely to have IDE. Granier et al. [26] found that AF patients with a large occluder in LAAO were more prone to delayed endothelialization and IDE, which is consistent with our finding, although there was no significant difference between the IDE group and CDE group in their study. The reason for a large LAA diameter and/or a large occluder on endothelialization may be related to the magnitude of the trauma and the longer time required for the new endocardium to replace fibrin on the occlusive surface.
Moreover, for the first time, the results of this study indicated that high levels of serum urea also affected the endothelialization of occlusion. Urea is a waste product of protein digestion and floats freely in the glomerulus [27]. High levels of serum urea can provide amino acids for liver gluconeogenesis and the synthesis of proteins that contribute to immune function, but the continued high catabolism of critically ill patients will lead to decreased immune function and increased mortality [28]. Therefore, it is often considered as a marker of tissue necrosis, protein catabolism and renal perfusion [29, 30]. At the same time, it can accelerate the coagulation process and lead to the formation of thrombus, and also damage the endothelial cells of blood vessels, and increase the precipitation of lipids and the production of oxygen free radicals. As a result, the mechanism may be used to explain the impact of urea on the IDE after LAAO.
The nomogram is a kind of graphical model for estimating specific outcomes or survival in association with certain risk factors. It is difficult to identify the patients in whom IDE is likely to occur in the absence of a prediction model. In this study, a nomogram model of IDE after LAAO was built based on four independent risk factors: MR, LAA diameter, occlusion size and serum urea. The C-index was 0.70 (95% CI: 0.552–0.849) and the C-index of verification was 0.708 (95% CI: 0.596–0.820), which suggested that the nomogram model has good accuracy and differentiation for IDE. We can conduct a customized risk assessment and guide treatment for high-risk IDE AF patients according to the nomogram model.
In the present study, although there was no statistically significant difference in the incidence of DRT between the IDE and CDE groups at 3-month follow-up, IDE had an increased risk for DRT, which was consistent with the previous reports [9–12]. It is necessary to further confirm the correlation between IDE and DRT through studies with larger sample sizes and longer follow-up times.
In conclusion, this study suggests that IDE of the occluder after LAAO in AF patients is related to a high level of serum urea level, MR, LAA diameter and large occluder size. We conduct a customized risk assessment and guide treatment for high-risk IDE AF patients according to the nomogram model.
This study only assessed the degree of endothelialization from the imaging presentation, which was not supported by pathological evidence. The sample size to establish the nomogram predictor model was small and requires further enhancements. Also, the geographical limitation should be considered.