Heart failure (HF) is a clinical condition characterized by debilitating symptoms, poor quality of life, frequent hospital admissions and reduced survival. It imposes a great economic burden on health systems worldwide, as its global prevalence is estimated to be around 2%, with a rising trend in recent years [1]. Coronary heart disease (CAD) is still the most common etiological factor for HF and the main cause of HF with reduced ejection fraction (HFrEF) and one of the main factors contributing to HF with preserved ejection fraction (HFpEF) [1].
In recent years the main principles and pharmacological targets for the therapy of HFrEF and HFpEF have been described. For HFrEF patients the modulation of five pathways is recommended to improve clinical outcomes, referring to angiotensin 2, norepinephrine, aldosterone, neprilysin and sodium-glucose transport proteins (SGLT) [1]. In the latest 2024 AHA/ACC [2] and 2023 ESC [3] guidelines, various medications have been introduced as first-line (angiotensin receptor neprilysin inhibitor-/angiotensin-converting enzyme (ARNI/ACE) inhibitors, β-blockers, mineralocorticoid receptor antagonists (MRA) and SGLT2 inhibitors (SGLT2i)) and second-line (iron replacement therapy, ivabradine, omecamtiv mecarbil, vericiguat and hydralazine/nitrate) therapies [4]. Recently the STRONG study [5] underlined the need for early initiation and up-titration of treatment in HF patients for reducing re-hospitalizations and congestion symptoms and improving quality of life. Additionally, the use of N-terminal brain natriuretic peptide (NTproBNP) seems to make it possible to evaluate even oligosymptomatic patients at risk, promoting up-titration of medical care [5]. However, accumulating evidence suggests that even after introducing all new therapies, residual cardiovascular (CVD) risk exists in HF patients under optimal tolerated medical treatment [1–3]. In addition, hyperlipidemia, although not common among non-ischemic HF patients, but still an important determinant of poor prognosis, has not been well studied. Particularly the role of hypolipidemic medication in the prognosis of HF has rarely been investigated and understood in relevant clinical trials (especially considering their large methodological limitations). In this commentary, we aimed to summarize scientific knowledge and highlight clinical considerations regarding the role of hypolipidemic treatment in HF patients.
Hyperlipidemia and treatment in HF patients
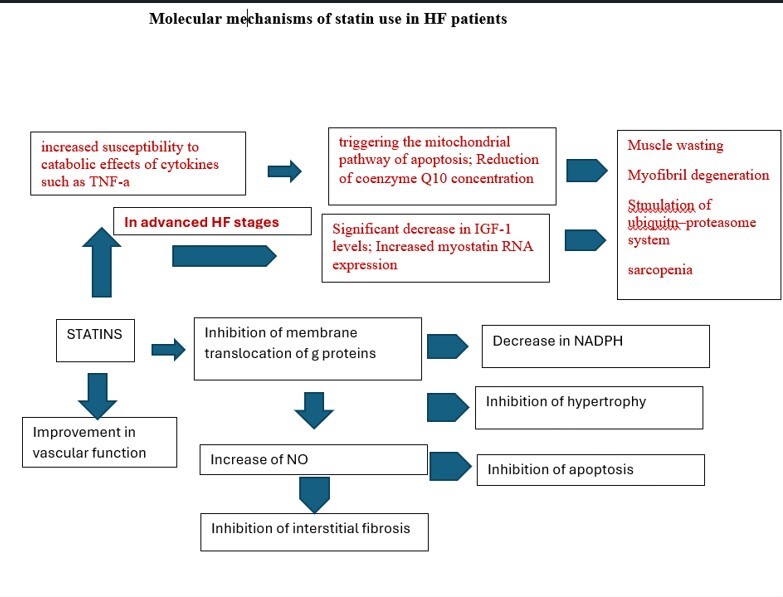
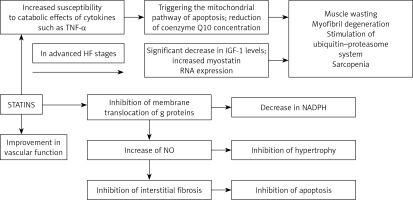
Heart failure, as the final pathway of clinical conditions that cause ventricular pressure and/or volume overload, is accompanied by hypertrophy, inflammation, angiogenesis, and apoptosis [1–3]. Statins may represent a potential treatment strategy for preventing cardiac hypertrophy and improving myocardial revascularization by decreasing nicotinamide adenine dinucleotide phosphate (NADPH) oxidation and activation, and increasing NO bioavailability. Thus, statins could be considered as a therapeutic approach that may prevent oxidation and increases relaxation and dilation. However, although statin therapy has proven to be highly effective in primary and secondary prevention of patients with dyslipidemias, studies in HF patients have shown inconclusive results. This is clearer in cases of advanced HF with deteriorated function of the left ventricle and clinical evidence of malnutrition or cachexia. That is the reason that lipid management in HF patients takes a modest position among all therapeutic tools, as a class III recommendation, which means that lipid-lowering therapy (LLT) initiation is not recommended in the absence of other indications (which in fact refers to only about 30% of all HF patients) [2, 3, 6–9]. This was mainly based on the results of previous large randomized controlled trials (RCTs), including patients with HFrEF [7, 8, 10, 11], as well as a meta-analysis of 24 RCTs that showed an insignificant benefit of statin treatment for CVD mortality in patients with HFrEF [9]. However, in the CORONA trial, even with its design limitations, based on retrospective sub-group analyses, rosuvastatin appeared to provide more CVD benefit in those with higher C-reactive protein (CRP) and lower NTproBNP, representing the profile of a patient who has not yet developed advanced HF [12, 13]. Nearly half of HF patients have HFpEF. Those patients show increased morbidity and mortality, while few pharmacological therapies have proved to improve survival. The pathophysiology of HFpEF is poorly understood but may involve a systemic proinflammatory state. Therefore, statins might improve outcomes in patients with HFpEF, especially in the context of primary and secondary prevention of atherosclerosis [14].
Statin use has been linked with Q10 mitochondrial depletion and muscle fatigue, while it reduced LDL cholesterol levels, leading to increased entry of lipopolysaccharide into cells, and increased inflammatory cytokine production [13, 15, 16]. Statins are known to suppress the prenylation of Rho protein and its downstream inflammatory cytokine production through NF-κB; thus, the effect of statins on inflammation is likely to vary depending on pathophysiological conditions. Based on pleiotropic effects, statins seem to act as immune suppressive agents and may have beneficial effects on those who have excessive and/or life-threatening immune-inflammatory reactions, such as in transplantations or HFpEF [17] (Figure 1).
In a meta-analysis that included 17 RCTs with 132,538 participants conducted over 4.3 years, statin therapy reduced LDL cholesterol levels by 0.97 mmol/l (38 mg/dl). Furthermore, numbers of patients experiencing non-fatal hospitalization due to HF were lower (RR = 0.90, 95% confidence interval (CI): 0.84–0.97) and the composite HF outcome (RR = 0.92, 95% CI: 0.85–0.99) but not HF death (RR = 0.97, 95% CI: 0.80–1.17) was also improved. The effect of statins on first non-fatal HF hospitalization was similar whether this was preceded by myocardial infarction or not [9]. Statin use in HF may also have pleiotropic effects as the modulation of Kv1.5 and Kv4.3 channel activity and the inhibition of sympathetic nerve activity change the myocardial action potential plateau and suppress arrhythmogenesis [15]. Moreover, the lipophilic atorvastatin showed a significant impact on all-cause mortality, left ventricular ejection fraction (LVEF), and hospitalization due to HF, although this was not obvious with hydrophilic rosuvastatin use [18]. Lipophilic statins are much more susceptible to drug interactions with many other medications metabolized by the CYP450 system. In two large RCTs rosuvastatin did not reduce the primary composite mortality/morbidity endpoints in HF patients with or without ischemic heart disease (IHD), although there was no increase in risk, and the number of hospitalizations was reduced [8, 11, 12].
There is some evidence suggesting that statins might reduce muscle strength and alter energy metabolism during aerobic exercise, leading to limited efficacy in HF patients, although their use may preserve or increase lean mass and exercise performance [19]. Statins might also rarely induce inflammatory myopathies characterized by significant elevations of enzymes levels, a myopathic pattern on the electromyogram, and inflammatory infiltrates evident on muscle biopsy, triggering the mitochondrial pathway of apoptosis [14, 20], although they regulate inflammation and improve cardiac sympathetic activity.
Advanced patient’s age and heart failure clinical stage have been related to statin intolerance, while in patients with less advanced HF, statin therapy might be beneficial in reduction of coronary events, whereas in severe HF, it could be too late for any potential benefits from statin therapy due to progressive loss of pump function. A recent meta-analysis based on the data from 4.2 million statin intolerance patients did not confirm the role of HF in the risk of statin intolerance [21].
PCSK9 is an emerging factor in HF patients, associated with energy metabolism disorders in heart failure [22]. PCSK9 can participate in cardiomyocyte apoptosis through NF-κB signal activation, induce autophagy of primary cardiomyocytes through reactive oxygen species, and take part in the immune process of tumors. It also participates in platelet activation and thrombosis by binding to platelet CD36 and takes part in the process of inflammation through the TLR4/NF-κB signaling pathway. The potential mechanism of PCSK9 regulating energy metabolism in cardiomyocytes during HF is mainly due to energy handling. When cardiomyocytes are exposed to a series of stimulations, the secretion of PCSK9 increases, activates PKB/Akt signaling and causes glucose metabolism disorder, affecting the fatty acid β-oxidation and tricarboxylic acid circulation in mitochondria. In addition, increased PCSK9 can also affect mitochondrial biogenesis, causing energy metabolism disorder of cardiomyocytes [1, 14, 18]. In the BIOSTAT-CHF cohort study [23], circulating PCSK9 was found to be significantly increased in patients with heart failure and positively correlated with mortality risk. However, in the ODYSSEY trial [24] the use of the PCSK9 inhibitor alirocumab in patients with a history of HF after an acute coronary event did not show any effectiveness in reducing the risk of major adverse cardiovascular events (MACE) and HF hospitalization. Emerging data in heart transplanted patients revealed that after 3 months of PCSK9 inhibitor initiation, there was an LDL reduction of 51.7% from baseline, while by 9 months, 24 out of 26 patients with available data had a recorded LDL level < 100 mg/dl. However, no interaction was observed between PCSK9 inhibitors and immunosuppressive medications [25]. Some considerations for the use of LLT in HF patients arose from the “cholesterol paradox”, in which low serum total cholesterol and lower high-density lipoprotein were associated with poor prognosis in patients with established HF (in contrast to patients without HF); however, this may represent a case of reverse causality. Additionally, lower lipid levels are evident in advanced HF patients, among whom hepatic congestion can impair hepatic biosynthesis of cholesterol, while intestinal congestion impairs cholesterol absorption. Thus, cachexia that accompanies advanced right heart failure is correlated with low LDL-C levels, poor nutritional status, and higher N-terminal pro-B-type natriuretic peptide levels. The effect of LVEF on the impact of statin treatment has been illustrated in previous studies. For example, in the PEARL study, pitavastatin use had a significant beneficial effect on patients with LVEF above 30% compared with those with LVEF < 30%, reflecting the interference of statins with catabolic pathways in advanced HF [26]. On the other hand, lipophilic statin may have a more beneficial effect compared to hydrophilic statins on cardiovascular mortality and morbidity, irrespectively of type of HF and level of LVEF, as has been illustrated in a meta-analysis of 17 studies [27]. New lipid-lowering treatments with bempedoic acid and obicetrapib may have an additive beneficial role in hyperlipidemic patients also exhibiting anti-inflammatory properties in metabolic syndrome, although studies on HF patients are still lacking.
Concluding remarks
All these considerations point to the fundamental role of lipid level modification in HF patients in the context of primary and secondary prevention of cardiovascular disease, avoiding discontinuation of lipid treatment in patients with newly diagnosed HF, the awareness that lipid metabolism play a crucial role in energy handling in HF and the acknowledgment that in patients with advanced heart failure and cachexia, supportive therapy may not include lipid treatment. In HFpEF patients, LLT can show beneficial effects in terms of secondary and even primary prevention for cardiovascular disease, again with limitations being noted in advanced stages of HFpEF [28]. Additionally, the initiation of new therapies in HF patients, such as the inhibition of sodium-glucose transport proteins in the nephron, has an important effect on the metabolic pattern in those patients, modifying residual metabolic risk beyond any lipid-lowering treatment (Table I).
Table I
Practical take-home message for the use of lipid-lowering therapy in heart failure patients