Introduction
The kidney, one of the most vital organs in the human body, plays a crucial physiological function of actively filtering excess fluid and eliminating waste products such as urea, uric acid, and creatinine [1]. The kidney maintains a balance of water, acid-base, and electrolytes through glomerular filtration and reabsorption in the proximal tubule [2]. However, ischemia, drug toxicity, immune injury, diabetes, and ecological factors can all cause kidney damage. Among them, kidney damage caused by ecological pollutants and occupational toxins has become a major public health concern. Chronic kidney disease (CKD) is defined as renal injury (determined based on urine tests, blood markers, or pathological abnormalities) and decreased renal function (glomerular filtration rate < 60 ml/min/1.73 m2) that persist for more than 3 months [3]. It is reported that the prevalence of CKD was approximately 11–13% worldwide in 2016 [4].
In the human body exposed to heavy metal pollutants, the kidney is a primary target for a variety of heavy metals. Cadmium and lead, the most toxic heavy metals, cause severe nephrotoxicity [5, 6]. Humans may come into contact with these metals from industrial occupations, the environment, diet, and lifestyle habits. Research has revealed that smoking and diet are the main pathways for cadmium and lead intake [7]. The half-life of cadmium in the kidney is 10–30 years, during which time it can cause substantial damage to the proximal tubule, thereby decreasing cadmium reabsorption [8]. Recent studies have revealed that exposure to lead increases the occurrence of kidney disease, but the mechanism by which lead enters renal cells is still unclear.
According to the Centers for Disease Control and Prevention of the United States (US), blood lead concentrations > 40 µg/dl can lead to proximal tubular dysfunction and a decreased glomerular filtration rate, contributing to interstitial and peritubular fibrosis. Ecological exposure to low levels of lead may be related to a slight decrease in renal function [9]. Orr et al. found that inorganic and organic compounds of mercury accumulate easily in the kidney [10], and almost all forms of mercury exert nephrotoxic effects. However, exposure to mercuric ion (Hg2+) conjugates causes the most serious renal damage [11]. Selenium and manganese are essential trace elements in the human body that are usually supplemented through food. The kidney is the organ with the highest selenium content. Some studies have indicated that selenium is involved in CKD progression [12], while others have reported conflicting findings regarding the relationship between selenium and CKD. A Mendelian randomization study on the involvement of selenium in CKD revealed that an increased selenium concentration is a risk factor for renal function damage [12], although further validation through a prospective cohort study is warranted. Manganese functions as a coenzyme in many biological processes. Although there is evidence suggesting that high-dose manganese is nephrotoxic [13], exposure to low-dose manganese may exert a protective effect against preeclampsia or kidney disease [14].
In the real world, humans are exposed to a variety of heavy metals, and various heavy metals have mutual interactions in and a cumulative effect on the human body. Therefore, studying the relationship between exposure to heavy metals and the occurrence of CKD is of great significance. Currently, there is limited research on the relationship between blood concentrations of heavy metals and the occurrence of CKD. Therefore, this study used data from the National Health and Nutrition Examination Survey 2011–2018 in the US to evaluate the relationships between blood concentrations of mercury, lead, cadmium, selenium, and manganese and the occurrence of CKD. The study findings may provide guidance for the prevention and control of heavy metal exposure to reduce the risk of CKD.
Material and methods
Data sources
The data for this study were obtained from the database of NHANES III, a health and nutrition survey led by the National Center for Health Statistics (NCHS) in the US, using a multi-stage probability sampling method. The NHANES includes US residents aged 2 months and above, and the outcomes can be extrapolated to the entire US population. Its advantage lies in the use of a combination of interviews and physical examinations such as medical examination, oral examination, physical measurements, and laboratory tests led by professional medical personnel to collect data on demographic and socioeconomic characteristics, diet, and health issues. The research protocol is approved yearly by the NCHS Research Ethics Review Committee, and signed informed consent is obtained from all participants.
Study population and variables
From the 39,156 participants included in NHANES III 2011–2018, 14,150 who lacked data on blood concentrations of mercury, lead, cadmium, arsenic, and chromium, 7,003 who lacked data on serum creatinine concentrations, 2,445 aged under 18, and 108 pregnant women were excluded. A total of 15,450 participants were included in the present study.
Data on the following parameters were extracted from the survey database: blood concentrations of five heavy metals (mercury, lead, cadmium, selenium, and manganese), age, sex, race (Mexican American, non-Hispanic Black, non-Hispanic White, and other races), education (under high school, high-school graduation, or above), marital status (married/living with partner, never married, widowed/divorced/separated), smoking status (lifetime smoking of more than 100 cigarettes), alcohol drinking status (never drinker, ever drinker, drinker), poverty income ratio (PIR), metabolic equivalent minutes (MET), body mass index (BMI), hypertension, hyperlipidemia, and diabetes. The flowchart of inclusion and exclusion of participants is provided in Figure 1.
Heavy metal measurements
In NHANES 2011–2018, whole blood samples were directly used, following an easy dilution and preparation step, for the measurement of mercury, lead, cadmium, selenium, and manganese concentrations by mass spectrometry.
Statistical analysis
All participants were divided into the CKD group and control group based on the serum creatinine concentration, and baseline demographic characteristics were compared between the two groups. Continuous variables that followed a normal distribution are represented as means ± standard deviations and were compared using a t-test. Categorical variables are represented as the number of cases and percentages and were compared using a χ2 test.
To explore the relationship between the risk of CKD and the blood concentrations of heavy metals, the sample population was divided into four groups based on the quartiles of heavy metal concentrations in blood (Q1: < 25th percentile, Q2: 25th–50th percentile, Q3: 50th–75th percentile, Q4: ≥ 75th percentile). Using the Q1 group as the reference group and controlling for confounding factors, namely income, education, race, sex, age, work, alcohol consumption, smoking, BMI, systolic blood pressure, and MET, a weighted logistic regression model was used to analyze the relationships between blood concentrations of heavy metals and the occurrence of CKD. The results are reported as p-values, 95% confidence intervals (CIs), and odds ratios (ORs). A restricted cubic spline model was used to evaluate the dose–response relationship. All statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC, USA), and p-values < 0.05 (two-tailed) were considered statistically significant.
Results
Participant characteristics
This study included 15,450 participants aged 18 and above from the NHANES III database. The mean age of the participants was 48.2 ±18.3 years; 7,861 were female (50.9%) and 7,589 were male (49.1%). Of the 15,450 participants, 2,925 had developed CKD and were included in the CKD group, while the remaining 12,525 were included in the control group (Table I). The sex ratio, education, and marital status were similar between the CKD and control groups (p > 0.05). Compared with the control group, the CKD group had more older participants, a higher proportion of non-Hispanic White individuals, a lower PIR, more smokers, a lower MET, a higher BMI, and more patients with hypertension, hyperlipidemia, and diabetes (Table I).
Table I
Baseline characteristics
Relationships between blood concentrations of cadmium, lead, mercury, selenium, and manganese and the occurrence of CKD
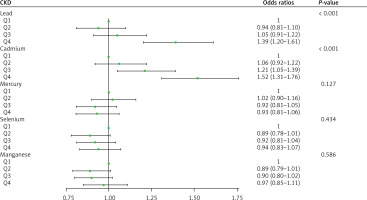
To explore the relationships between blood concentrations of individual heavy metals and the occurrence of CKD, the heavy metal concentrations were divided into quartiles, and the lowest quartile Q1 was used as the reference group (Figure 2). After adjustments for age, sex, ethnicity, education, marital status, and PIR, smoking, drinking, BMI, diabetes, hypertension, creatinine, hyperlipidemia, and MET, with regard to blood lead concentrations, the ORs for CKD in Q2, Q3, and Q4 relative to Q1 were 0.94 (95% CI: 0.81–1.10), 1.05 (95% CI: 0.91–1.22), and 1.369 (95% CI: 1.20–1.61), respectively. The occurrence of CKD in the Q4 group was significantly higher than that in the Q1 group (p < 0.001). With regard to blood cadmium concentrations, the ORs for CKD in Q2, Q3, and Q4 relative to Q1 were 1.06 (95% CI: 0.92–1.22), 1.21 (95% CI: 1.05–1.39), and 1.52 (95% CI: 1.31–1.76), respectively. The occurrence of CKD in the Q4 group was significantly higher than that in the Q1 group (p < 0.001).
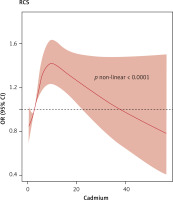
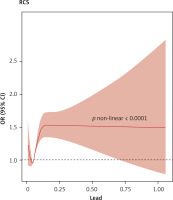
With regard to blood mercury, selenium, and manganese concentrations, the occurrence of CKD in the Q2, Q3, and Q4 groups was not different from that in the corresponding Q1 groups (p > 0.05). To further explore the dose–response relationship between blood cadmium and lead concentrations and the occurrence of CKD, we adjusted for confounding factors and plotted a restricted cubic spline curve. Figures 3 and 4 show non-linear relationships between blood cadmium and lead concentrations and the occurrence of CKD (p < 0.001). With the increase in the blood lead concentration, the occurrence of CKD first decreased, then rapidly increased, and then plateaued after reaching a certain concentration. With the increase in the blood cadmium concentration, the occurrence of CKD first rapidly increased and then gradually decreased.
The interaction effects between blood concentrations of cadmium, lead, mercury, selenium, and manganese and individual characteristics on CKD
The results of multivariate analysis revealed that there was an interaction effect between the blood cadmium concentration and age, BMI, and blood pressure. All results are reported in comparison with the Q1 groups as the reference. Among people aged 60 years and above, the occurrence of CKD in the Q3 and Q4 groups was 1.3 (95% CI: 1.05–1.6) and 2.06 (95% CI: 1.64–2.57), respectively. In individuals with obesity (BMI ≥ 25 kg/m2), the occurrence of CKD in the Q3 and Q4 groups was 1.36 (95% CI: 1.16–1.59) and 1.66 (95% CI: 1.4–1.97), respectively. In individuals with hypertension, the occurrence of CKD in the Q3 and Q4 groups was 1.31 (95% CI: 1.08–1.59) and 1.84 (95% CI: 1.5–2.26), respectively.
Interaction effects were also observed between the blood lead concentration and age, BMI, and blood pressure. Among individuals aged 60 years and above, the ORs for CKD in the Q3 and Q4 lead groups were 1.45 (95% CI: 1.13–1.86) and 1.85 (95% CI: 1.44–2.37), respectively. Among obese individuals (BMI ≥ 25 kg/m2), the ORs for CKD in the Q3 and Q4 lead groups were 1.11 (95% CI: 0.94–1.31) and 1.52 (95% CI: 1.29–1.8), respectively. Among individuals with hypertension, the ORs for Q3 and Q4 lead concentrations were 1.36 (95% CI: 1.1–1.68) and 1.72 (95% CI: 1.39–2.13), respectively. No interaction effects were observed between the blood concentrations of mercury, selenium, and manganese and individual characteristics in the Q4, Q3, and Q2 groups relative to the corresponding Q1 groups in terms of increased occurrence of CKD (Supplementary Table SI).
Discussion
Heavy metals have toxic effects on multiple organs in the human body. This study analyzed the relationship between blood concentrations of mercury, lead, cadmium, selenium, and manganese and the occurrence of CKD using the data of adults included in NHANES 2011–2018. The results showed that higher blood cadmium and lead concentrations are associated with increased occurrence of CKD, and both exhibit a non-linear dose–response relationship with CKD. In addition, blood cadmium and lead showed interaction effects with three individual characteristics, namely older age, hypertension, and obesity, suggesting that these characteristics increase the occurrence of CKD.
Multiple studies have revealed that oxidative stress plays a key role in the development of CKD. In vitro studies have revealed that both lead and cadmium can cause oxidative stress in rat kidneys or renal tubular epithelial cells [15]. Exposure to lead can increase lipid peroxidation and phospholipid degradation in kidney cells, contributing to cell nephrotoxicity and loss of membrane integrity [16]. Cross-sectional studies from Spain, South Korea, and the US have identified exposure to cadmium as a risk factor for CKD [17–19]. Wu et al. [20] reported that the higher the blood concentrations of cadmium and lead were, the higher was the risk of developing CKD. Nationwide research in the US showed that the presence of cadmium and lead in blood reduced the glomerular filtration rate and increased protein excretion in urine [21]. An epidemiological study of 14,778 American adults (≥ 20 years old) revealed that even when the blood concentrations of cadmium and lead were low, increased protein excretion in urine and decreased glomerular filtration rates were observed [22]. Hsueh et al. [23] observed that an increase in blood cadmium and lead concentrations gradually increased the OR for CKD.
Our study also revealed that the Q4 group with the highest blood cadmium and lead concentrations had increased occurrence of CKD compared with the Q1 group, which is consistent with the literature. The restricted cubic spline plot shows that the occurrence of CKD first decreases and then rapidly increases with the increase in blood lead concentrations and then ultimately plateaus, while it first increases and then decreases with the increase in blood cadmium concentrations. Consistently, Yuebin et al. [24] found that the occurrence of CKD increased with increasing blood cadmium concentrations.
Selenium is an important micronutrient for mammals and an essential component of selenoproteins, the dysfunction of which causes oxidative damage [25]. However, previous studies have reported conflicting findings regarding the relationship between selenium and CKD. A study conducted in Taiwan reported that high blood selenium concentrations significantly reduced the OR for CKD and increased the glomerular filtration rate [20]. Our findings did not reveal an evidence-based significant relationship between the blood selenium concentration and the occurrence of CKD. Similarly, no significant relationship was found between blood mercury and manganese concentrations and the occurrence of CKD. Further research is warranted to explore these potential associations.
The present study showed that Q3 blood cadmium and lead concentrations increased the occurrence of CKD in individuals aged over 60, those with obesity, and those with hypertension. Age is an independent risk factor for CKD, and lead and cadmium are both mutually interactive toxins that have cumulative effects. The health risks posed by exposure to these heavy metals increase with age. For example, the occurrence of CKD increases with the increase in blood cadmium and lead concentrations. Obesity and hypertension are risk factors for a variety of chronic diseases. A study based in Taiwan showed that the OR for CKD in Chinese individuals with hypertension was significantly higher than that in individuals without hypertension (OR = 3.06, 95% CI: 2.17–4.32) [20]. Kim et al. [26] found a significant relationship between the risk of CKD and elevated blood cadmium concentrations in adults with hypertension, with an OR of 1.52 (95% CI: 1.05–2.19, p = 0.026). These reports are consistent with our findings. Numerous studies have explored the relationship between metal exposure and the risk of CKD events. In particular, the concentration of heavy metals in human blood or urine has been found to be closely associated with soil heavy metal levels, particularly in industrializing countries. This correlation is significant due to the widespread exposure to lead (Pb), mercury (Hg), and cadmium (Cd) through air, food, cigarettes, gasoline, contaminated crops, and seafood, resulting in chronic exposure to low levels of these metals in the current environment [27]. Importantly, these metals have biologic half-lives on the order of decades, and as individuals age, long-term accumulation and retention of these metals in the body can lead to excessive buildup in certain tissues, particularly the kidneys, disrupting normal physiological functions. Extensive experimental evidence supports the notion that exposure to heavy metals can induce oxidative stress, inflammation, and lipid peroxidation in organs [28, 29].
A study conducted on lead exposure has revealed a potential link between lead exposure and obesity. This may be attributed to the impact of lead on the hypothalamic-pituitary-adrenal (HPA) axis, specifically altering adrenocortical responses to acute stress. Notably, it was observed that individuals with low blood lead levels displayed altered responses of adrenocortical activity, whereas those with high blood lead levels exhibited significantly elevated cortisol responses. Furthermore, lead exposure itself was found to elicit a stress-like response by increasing adrenocorticotropic (ACTH) hormone and corticosterone concentrations, thereby indicating dysregulation of the HPA axis caused by lead exposure. It is noteworthy that studies have also demonstrated that lead exposure can trigger the generation of reactive oxygen species while inhibiting their scavenging and neutralization through antioxidant defense mechanisms. Consequently, this oxidative stress coupled with abnormalities in fat metabolism may establish a vicious circle, potentially leading to obesity. Hence, it is reasonable to suggest that lead exposure may contribute to obesity, partially through the induction of oxidative stress. Additionally, it is worth mentioning that the mechanism of obesity induced by cadmium exposure may also involve its ability to induce oxidative stress [30–32].
An association was found between Cd exposure and elevated blood pressure and an increased risk of hypertension in a recent study. Cd is believed to have direct effects on blood vessels, specifically inhibiting endothelial nitric oxide synthase and reducing acetylcholine-induced vascular relaxation, thereby leading to hypertension [33]. Additionally, Cd has been shown to enter lysosomes in renal cells and subsequently be released into the cytosol, causing damage to the renal tubules. This phenomenon can result in salt retention, volume overload, and ultimately hypertension [34]. Various studies have proposed a hypothesis regarding how lead exposure may affect blood pressure. They suggest that lead exposure leads to oxidative stress, limiting the availability of nitric oxide, increasing systemic vascular resistance, and altering the activity and production of hormones that regulate vascular tone [35, 36]. Multiple studies have revealed that many heavy metals have individual or cumulative effects on the kidneys. Future studies should investigate the cumulative effects of heavy metals to further elucidate the relationships between heavy metals and the occurrence of CKD [37, 38].
The advantage of this study is the use of the database of NHANES, which has a large sample size, standardized research protocol, and data representative of the US population. However, the variables examined varied across the different years analyzed in this study. Specifically, the data on blood concentrations of metal variables in the NHANES database were only accessible from 2011 to 2018. Consequently, the NHANES cohort spanning from 2011 to 2018 was chosen as the appropriate sample for conducting the study in question. Also, since the NHANES database contains cross-sectional research data, the causal relationships between elevated blood heavy metal levels and CKD could not be explored. A prospective study is warranted to infer causal relationships. In addition, ecological exposure to heavy metals was considered in terms of blood concentrations of heavy metals. Blood heavy metal concentrations are the most widely used indicators of chronic ecological exposure in population-based studies, although blood cadmium and lead concentrations may also be considered markers of acute short-term ecological exposure. We plan to conduct prospective large-scale studies to evaluate the causal relationships between ecological exposure to heavy metals and the occurrence of CKD.
In conclusion, our study establishes a correlation between elevated blood concentrations of cadmium and lead and a higher prevalence of CKD. Furthermore, we observed a non-linear dose-response relationship between both metals and CKD. Given the alarming levels of metal pollution in China and the escalating global burden of CKD, our findings suggest potential avenues for intervention strategies aimed at preventing and mitigating the impact of CKD in the general population. Consequently, these data hold substantial public health significance.
The results of this study showed that the occurrence of CKD increased with increasing blood concentrations of cadmium and lead, following a non-linear dose–response relationship. Our study also found interaction effects between blood cadmium and lead concentrations and advanced age, obesity, and hypertension, suggesting that these three variables increase the risk of CKD posed by high blood concentrations of cadmium and lead. Further research is needed to confirm the relationships between blood selenium, mercury, and manganese and the occurrence of CKD.