Introduction
Dyslipidemia is a leading risk factor for coronary heart disease (CHD) [1, 2], but its effects on the pathogenesis of stroke are less clear [3–10]. There are some inconsistencies regarding the relationship between cholesterol and ischemic stroke [3–9]. A meta-analysis of 900,000 individuals from 61 prospective cohort studies did not find any significant relationship between total cholesterol and fatal stroke risk [10]. However, risk of the first stroke was reduced in statin trials that included populations at high risk of vascular events; this effect was associated with lowering low-density lipoprotein cholesterol (LDL-C) levels [11–13]. In a meta-analysis of 65,138 individuals with 2282 strokes (204 hemorrhagic, 1565 ischemic, 513 unknown type), a significant proportional reduction in the incidence of the first stroke of any type was observed among participants treated with lipid-lowering agents (LLAs) [14]. There are several factors that may account for these conflicting results. First, stroke is a heterogeneous condition with various etiologies; so lipid abnormalities may be related to some subtypes of stroke but not others [15]. Secondly, lipo-protein sub-fractions have different impacts on stroke risk [16]. The association between LDL-C and different types of strokes, therefore, needs to be more carefully evaluated.
The impact of lipid-lowering medications on stroke types has not been well established and is still a matter of considerable debate [17]. Although there is some evidence with regard to the effectiveness of lipid-lowering agents on the risk of stroke (mainly ischemic) [18–21], some trials in which individuals were treated with non-statin therapy showed no (significant) reduction in the risk of stroke [22]. Moreover, a meta-analysis of statin trials reported a non-significant association between statin therapy and the risk of hemorrhagic stroke [23]. In another systematic review and meta-analysis of non-statin clinical trials aiming for further LDL-C lowering, a non-significant positive association between LDL-C lowering and increased risk of hemorrhagic stroke was reported [24]. Recent randomized controlled trials (RCTs) demonstrated that statins significantly decrease vascular events in primary and secondary prevention of myocardial infarction [25–31]. A reduction in the risk of strokes (ischemic, transient ischemic attack (TIA) and brain hemorrhage) was reported in only 3 of these trials [25, 26, 29]. Recent trials with PCSK9 inhibitors suggested a significant association between combination therapy and reduced risk of ischemic stroke, and a lack of significant difference in hemorrhagic stroke between groups [32, 33].
Due to the paucity of studies and conflicting findings, we performed the present analysis to obtain more insights into LDL-C in relation to different types of stroke events based on observational studies. We also performed a systematic search and meta-analysis on the impact of LLAs and risk of different types of stroke based on RCTs; finally, we applied trial sequential analysis (TSA) to determine whether the pooled trial data provided sufficient evidence to reach a reliable conclusion regarding the effect of LLAs and risk of different types of stroke.
Material and methods
Cohort studies
Literature search
The meta-analysis was reported according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [34]. The primary exposure of interest was LDL-C level while the primary outcomes were different types of stroke. Prospective cohort studies published up to 1 September 2019 without language restriction were searched using PubMed, Embase, and Scopus databases; the query syntax of PubMed is shown in Supplementary Table SI. This was complemented by additional searches of the reference list of eligible articles, and email correspondence with authors for additional data where relevant.
After excluding duplicates and based on titles and abstracts, we excluded studies in animals, and in those < 18 years old. Eligible studies were selected by using predefined inclusion criteria of prospective cohort studies, and original articles on the association of LDL-C and stroke risk. In addition, a supplementary search of reference lists of previous reviews or meta-analyses was conducted.
Study selection
Titles and abstracts were screened by two reviewers (MM and NS). To avoid selection bias, the reviewers were blinded to the names, qualifications and institutional affiliations of the study authors. The agreement between the reviewers was excellent (κ index: 0.90; p < 0.001). Disagreements were resolved at a meeting between the authors prior to selected articles being retrieved.
We included studies if they met all the following criteria: (1) exposure was LDL-C; (2) population-based cohort studies and reported stroke risk data; (3) relative risk (RR), hazard ratio (HR) or odds ratio (OR) estimates with 95% confidence interval (CI) adjusted for multivariable factors were available or could be calculated. Narrative reviews, comments, opinion pieces, methodological reports, editorials, letters or any other publications lacking primary data and/or explicit method descriptions were excluded.
Risk of bias assessment and data extraction
Full texts meeting the inclusion criteria were retrieved and screened to determine eligibility by two reviewers (MM and NS). The study risk of bias assessment was performed according to the Newcastle-Ottawa Scale (NOS, Supplementary Table SII) [35]. By evaluation of selection, comparability and outcome, the NOS scores studies from 0 (highest degree of bias) to 9 (lowest degree of bias). Additionally we investigated the funding sources of all of the eligible studies. Following assessment of methodological quality, two reviewers (MM and NS) extracted data using a purpose-designed data extraction form and independently summarized the most important results from each study. These summaries were compared and any differences of opinion resolved by discussion and consultation with a third reviewer (MB). Any further calculations on study data considered necessary were conducted by the first reviewer and checked by the second reviewer. Information extracted from each eligible study included the following items: author, year and references, study name, % of men, mean age, follow-up time (years), number of individuals overall, categories of LDL-C levels, number of individuals per LDL-C category, main confounders, number of strokes per LDL-C category, type of strokes.
Statistical analysis
For studies that reported results from different multivariable-adjusted models, the model with the most confounding factors was extracted for analyses. The random-effect model with inverse variance method was applied to calculate pooled RRs, 95% CI and p-value for heterogeneity. RRs comparing the highest category of LDL with the lowest category of LDL were combined across studies to generate the summary associations. The extent of heterogeneity across studies was examined using the I2 test [36–38] and an I2 > 50% together with two-sided p < 0.05 indicated significant heterogeneity [36–38].
Publication bias
Potential publication bias was explored using visual inspection of Begg’s funnel plot asymmetry, Begg’s rank correlation and Egger’s weighted regression tests. The Duval and Tweedie trim method was used to adjust the analysis for the effects of publication bias [39]. Meta-analysis was conducted using Comprehensive Meta-Analysis (CMA) V3 software (Biostat, NJ) [40].
Randomized controlled trials
Literature search
We reported this study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines [41, 42]. We searched multiple databases, including PubMed/Medline, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), and Web of Science by Clarivate and www.clinicaltrials.gov register until 1st September 2019 using a combination of search terms (Supplementary Table SIII). This was complemented by a hand search of the reference list of eligible articles, and email correspondence with authors for additional data where relevant.
Study selection
We included RCTs evaluating the effect of pravastatin, lovastatin, atorvastatin, simvastatin, fluvastatin, cerivastatin, rosuvastatin, pitavastatin, HMG-CoA reductase inhibitor, statin, ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, alirocumab, evolocumab or bococizumab on the outcomes of interest. Eligible studies had to meet the following criteria: (1) an RCT with either parallel or crossover design, (2) studies of patients treated with mentioned agents compared with a control group (either with no lipid-lowering agents or placebo), and, (3) containing sufficient information on the primary outcome at end of follow-up in each group. The primary outcome was stroke.
Exclusion criteria were: (i) non-clinical studies, (ii) observational studies with case-control, cross-sectional or cohort design, (iii) sample size < 2000 participants, and (iv) duration of treatment < 2 years. Narrative reviews, comments, opinion pieces, methodological, editorials, letters or any other publications lacking primary data and/or explicit method descriptions were also excluded. Study selection started with the removal of duplicates; then titles and abstracts were screened by two reviewers (MM and NS). To avoid selection bias, they were blinded to the names, qualifications and institutional affiliations of the study authors. The agreement between the reviewers was excellent (κ index: 0.92; p < 0.001). Disagreements were resolved at a meeting between reviewers prior to selected articles being retrieved.
Data extraction
Full texts were retrieved and screened to determine eligibility by two reviewers (MM, NS). Following assessment of methodological quality, the same two reviewers extracted data into a purpose-designed data extraction form, and independently summarized what they considered to be the most important results from each study. These summaries were compared and any differences of opinion were resolved by discussion and consultation with the third reviewer (MB). Any further calculations on study data considered necessary were conducted by the first reviewer (MM) and checked by the second reviewer (NS). Descriptive data extracted included author(s) and references, study name, year of publication, country of origin, men (%), mean age, mean follow-up (years), number of participants, type of intervention, type of control, number of participants per drug arm, baseline LDL cholesterol level, achieved LDL cholesterol level, strokes per drug arm, types of strokes, main confounders.
Risk of bias assessment
A systematic assessment of bias in the included RCTs was performed using the Cochrane tool [43]. The evaluated items were: adequacy of random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessment, handling of drop-outs (incomplete outcome data), selective outcome reporting, and other potential sources of bias. Each item was judged as low risk, high risk or unclear risk (Supplementary Table SIV).
Statistical analysis
A DerSimonian-Laird random effects model and the generic inverse variance method were used to evaluate the effect of LLAs on stroke [43]. Heterogeneity was quantitatively assessed using the I2 index. In order to evaluate the influence of each study on the overall effect size, sensitivity analysis was conducted using the leave-one-out method, i.e. removing one study each time and repeating the analysis [43]. The main effect measure was the RR and its 95% CI. We also calculated the number needed to treat (NNT). NNT is the number of individuals required to experience the intervention in order to avoid one stroke.
We performed the following meta-analyses: (1) by levels of achieved LDL-C (achieved LDL-C level ≤ 1.3 mmol/l (50 mg/dl), 1.3 mmol/l (50 mg/dl) to < 1.8 mmol/l (70 mg/dl), and ≥ 1.8 mmol/l/70 mg/dl); (2) by statin or non-statin group (statin trials vs. non-statin trials), (3) by primary vs secondary prevention, (4) separately by specific type of stroke: ischemic and hemorrhagic.
To reduce the risk of type I error caused by pooling data from the same trials or from trials with missing data, trial sequential analysis (TSA) was applied. Interim analysis of a single randomized trial avoids type I error by creating monitoring boundaries for an estimated difference between groups, so if the estimated difference is reached the trial could be terminated. TSA uses a similar accurate method to create monitoring boundaries and estimate the optimal sample size in meta-analyses. TSA performs a cumulative meta-analysis with the results of the available studies (represented by the Z-curve): as each new study is included, significance is tested and CIs are estimated. It also creates adjusted boundaries for benefit, harm, and futility, and estimates the optimal sample size for a given difference between treatment arms, so that a smaller estimated difference would result in wider boundaries and a greater optimal sample size. If one of the boundaries (benefit, risk or futility) or if the optimal sample size is reached, firm conclusions might be made (for that predefined difference) and further studies are deemed unnecessary; instead, if no boundaries are reached, further studies are needed to settle the question. Random errors were accounted for by calculating a diversity-adjusted required information size, which represented monitoring boundaries to determine whether the evidence in our cumulative meta-analysis was sufficient to reach a conclusion. It is also adjusted for the variability between trials and for the amount of available evidence. The required sample size for the TSA was estimated using two-side testing, α = 0.05 (two-sided), β = 0.20 (power of 80%), the incidence rate in the control group, and 35% relative risk reduction (RRR) in the LDL-lowering agents intervention group. Sensitivity analysis was conducted with 20% relative risk reduction in the LDL-lowering agents intervention group. TSA was conducted using TSA version 0.9 beta (Copenhagen Trial Unit, Copenhagen, Denmark; available at www.ctu.dk/tsa).
Ethics
This investigation uses published or publicly available summary data. No original data were collected for this manuscript. Ethical approval for each of the studies included in the present analysis can be found in the original publications (including informed consent from each participant). The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Results
Cohort studies
Characteristics of the included studies
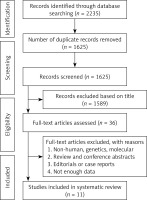
Of 36 eligible full articles, 11 cohort studies were finally included in the meta-analysis (Figure 1). In total 355,591 participants with 11,888 events including stroke (3 studies), ischemic stroke (6 studies), hemorrhagic stroke (6 studies), intracerebral hemorrhage (2 studies), intraparenchymal hemorrhage (2 studies), cerebral infarction (1 study) and cerebral hemorrhage (1 study) were entered in the analysis. All studies included both sexes and two of them presented sex-specific results. The mean follow-up duration was 9.9 years (range: 4.9–19 years) (Table I).
Table I
Characteristics of 11 prospective cohort studies on LDL-C and stroke
| Author, year, and references | Study name | Men (%) | Mean age | Mean follow-up[years] | No. of cases | No. of subjects | Outcome | Reference | Main confounders |
|---|---|---|---|---|---|---|---|---|---|
| Amarenco et al. [44] | SPARCL | – | – | 4.9 | 491 | 4731 | Stroke Ischemic stroke Hemorrhagic stroke | First tertile (median = 110 mg/dl) | – |
| Glasser et al. [46] | REGARDS | 45 | – | 6.9 | 817 | 24098 | Ischemic stroke (749) Hemorrhagic stroke (68) | – | Age, race, gender, education, region, and income, SBP, hypertensive medication use, lipid-lowering medication use, smoking, atrial fibrillation, left ventricular hypertrophy, history of heart disease, and diabetes |
| Holme et al. [49] | AMORIS | – | 30–85 | 11.8 | 5900 | 148600 | Ischemic stroke Hemorrhagic stroke | – | Age, AMI, diabetes and hypertension |
| Imamura et al. [45] | Hisayama | 42 | – | 19 | 271 | 2351 | Stroke Ischemic stroke Hemorrhagic stroke | First quartile (≤ 2.65 mmol/l) | Age, sex, HDL cholesterol, triglycerides, systolic BP, ECG abnormalities, fasting blood glucose, BMI, current drinking, current smoking, and regular exercise |
| Ma et al. [51] | Kailuan | – | 51.3 | 9 | 753 | 96043 | Intracerebral hemorrhage | < 1.3 mmol/l | Age; sex; smoking; alcohol intake; education; physical activity; average monthly income of each family member; salt intake); updated diabetes status; use of antihypertensive, lipid-lowering agents, aspirin, and anticoagulants; updated cumulative average body mass index; triglycerides, high-density lipoprotein cholesterol, and alanine aminotransferase levels; systolic blood pressure; diastolic blood pressure; and estimated glomerular filtration rate |
| Nakaya et al. [52] | J-LIT | 31.6 | 57.7 ±7.9 | 6 | 220 | 41088 | Cerebral infarction Cerebral hemorrhage | – | – |
| Noda et al. [73] | The Ibaraki Prefectural Health Study | 33.8% | 58.6 | 10.3 | 264 | 91219 | Death due to intraparenchymal hemorrhage | – | Body mass index (sex-specific quintiles), blood pressure categories (normal, mild hypertension, moderate hypertension, or severe hypertension), antihypertensive medication use (yes or no), lipid medication use (yes or no), diabetes status (normal, impaired glucose tolerance, or diabetes mellitus), γ-glutamyl transferase (sex-specific quintiles), kidney dysfunction (yes or no), smoking status (never, ex-smoker, and current smokers of 1 to 19 or ≥ 20 cigarettes/day), alcohol intake category (never or ex-drinkers, occasional drinkers, and habitual drinkers of < 69 and ≥ 69 g of alcohol per day) |
| Psaty et al. [47] | The Cardiovascular Health Study | 33.18 | – | 7.5 | – | 5888 | Ischemic stroke Hemorrhagic stroke | < 100 mg/dl | Age, sex, diabetes mellitus, smoking status, cardiovascular disease, and systolic blood pressure |
| Stoekenbroek et al. [48] | EPIC-Norfolk prospective population study | 43.9 | 59 | 12.1 | 3087 | 21798 | Ischemic stroke Hemorrhagic stroke | < 3.24 mmol/l | Age, sex, smoking, BMI, diabetes, HDL-c, and systolic blood pressure |
| Sturgeon et al. [97] | The Atherosclerosis Risk in Communities Study (ARIC) and the Cardiovascular Health Study (CHS) | 44.8/44.2 | 54.2/72.8 | 13.5 | 135 | 15792/5888 | Intracerebral hemorrhage (ICH) | – | – |
| Wieberdink et al. [50] | Rotterdam | – | – | Median 9.7 | 85 | 10994 | Intracerebral hemorrhage | Quartile 1 = (0.1–3.2) mmol/l | Age, sex, lipid-lowering medication use, systolic blood pressure, blood pressure-lowering medication use, diabetes mellitus, serum glucose level, current cigarette smoking, body mass index, antithrombotic medication use, alcohol intake and subcohort |
We analyzed different types of stroke separately: any stroke [44–46], hemorrhagic stroke [44–49], ischemic stroke [44–49], intracerebral hemorrhage [50, 51], deep or infratentorial microbleeds [50], cerebral infarction [52] and cerebral hemorrhage [52].
Risk of bias assessment
Results of risk of bias assessment are shown in Supplementary Table SII, with 8 studies scoring ≥ 8, and three studies with a score of 7.
Association between LDL-C levels and stroke
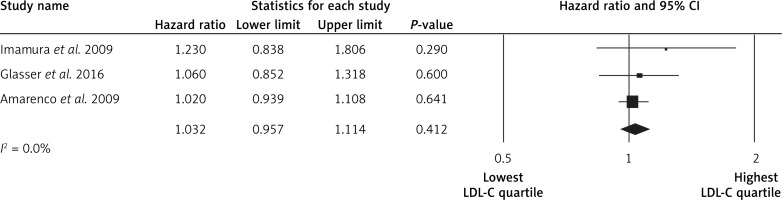
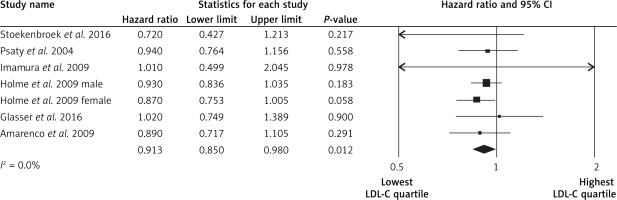
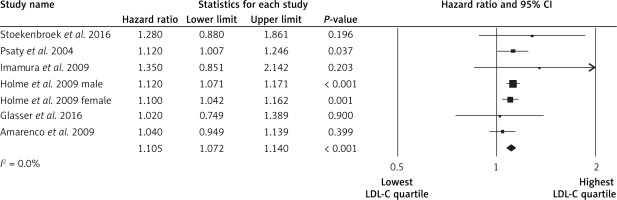
The highest LDL-C quartile was not associated with stroke events in comparison to the lowest LDL-C quartile (HR = 1.03, 95% CI: 0.95–1.11, p = 0.4, n = 3 studies, Figure 2), with low heterogeneity of effects (I2 = 0%, p = 1.0). Subjects in the highest LDL-C quartile had a lower risk of the hemorrhagic strokes (HR = 0.91, 95% CI: 0.85–0.98, p = 0.012, n = 7 studies, Figure 3), with low heterogeneity across studies (I2 = 0%, p = 0.9). Subjects in the highest LDL-C quartile had a higher risk of ischemic stroke (HR = 1.11, 95% CI: 1.07–1.14, p < 0.001, n = 7 studies, Figure 4), with low heterogeneity among studies (I2 = 0%, p = 0.7). No significant association was found between LDL-C and intracerebral hemorrhage events (HR = 0.99, 95% CI: 0.77–1.28, p = 1.0, n = 2 studies; I2 = 0%, p = 0.7).
Sensitivity analysis
In leave-one-out sensitivity analyses, the pooled effect estimates were similar for the association between LDL-C and hemorrhagic stroke (HR = 0.91, 95% CI: 0.85–0.98) and for the association between LDL-C and ischemic stroke (HR = 1.10, 95% CI: 1.07–1.14). This stability confirms that the significant difference between the studied groups is the overall effect of all included studies.
Publication bias
Regarding the association between LDL-C and hemorrhagic stroke, Egger’s linear regression indicated absence of publication bias (intercept = –0.55, 95% CI: –6.91, 6.00, p = 0.837); also, Begg’s rank correlation test (Kendall’s τ with continuity correction = 1.00, z = 0.244, two tailed p = 0.806) did not indicate publication bias.
RCTs
Study selection
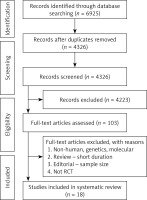
A flowchart of study selection is shown in Figure 5; a total of 6925 unique citations were identified from searches, of which 2599 records remained after removing duplicates. After screening via titles and abstracts, 103 articles remained for further evaluation, of which 18 RCTs with 165,988 participants were included in the meta-analysis.
Characteristics of included trials
A summary of trial characteristics is presented in Table II. The included trials were published between 2002 [53] and 2018 [32] and were performed in the United States of America (one study) [54], the UK (one study) [27] and Italy (one study) [55]. The other 15 studies were multicenter [29, 30, 32, 33, 53, 56–64]. The number of study participants ranged from 2107 [56] to 27,564 [33]. The proportion of men in the studies ranged from 48.3% [29] to 82.2% [54]. The mean age (63.1 years) of participants ranged from 49.7 [56] to 75.3 [55] years. The follow-up duration from the baseline to endpoint across studies was from 2.2 year [33] to 6 years [64]) (mean follow-up was 4.1 years). Baseline and achieved LDL-C level were assessed. Baseline LDL-C level ranged from 2.25 [32] to 4.1 (mmol/l) [56], and the range of achieved LDL-C level was from 0.78 [33] to 3.1 (mmol/l) [56].
Table II
Characteristics of included trials on lipid-lowering agents and stroke
| Author and references | Study name, year of publication, country of origin | Men (%) | Mean age | Mean follow-up [years] | Number of participants | Baseline LDL cholesterol level [mmol/l] | Achieved LDL cholesterol level [mmol/l] |
|---|---|---|---|---|---|---|---|
| Furberg C et al. [53] | ALLHAT-LLT, 2002, multicenter | 51.1 | 66.3 | 4.8 | 10355 | 3.77 | 2.87 |
| HPS (Cohort 1) [27] | HPS(p), 2002, UK | – | – | 5 | 17256 | – | 2.3 |
| HPS (Cohort 2) [27] | HPS(s), 2002, UK | – | – | 4.8 | 3280 | – | 2.4 |
| Shepherd J et al. [29] | PROSPER, 2002, multicenter | 48.3 | 75.3 | 3.2 | 5804 | 3.8 | 2.5 |
| Holdaas H et al. [56] | ALERT, 2003, multicenter | 66 | 49.7 | 5.1 | 2107 | 4.1 | 3.1 |
| Sever PS et al. [30] | ASCOT-LLA, 2003, multicenter | 81.1 | 63.1 | 3.3 | 10305 | 3.4 | 2.21 |
| Koren MJ et al. [54] | ALLIANCE, 2004, centers in US | 82.2 | 61.1 | 4.3 | 2442 | 3.81 | 2.46 |
| Colhoun HM et al. [103] | CARDS, 2004, Ireland, UK | 68 | 61.6 | 3.9 | 2838 | 3.04 | 1.75 |
| Pedersen TR et al. [57] | IDEAL, 2005, multicenter | 80.8 | 61.6 | 4.8 | 8888 | 3.15 | 2.01 |
| LaRosa JC et al. [58] | TNT, 2005, multicenter | 81 | 61 | 4.9 | 10001 | 2.51 | 1.99 |
| Knopp RH et al. [59] | ASPEN, 2006, multicenter | 66.3 | 61 | 4 | 2410 | 2.93 | 2.04 |
| Amarenco P et al. [60] | SPARCL(s), 2006, multicenter | 59.6 | 62.7 | 4.9 | 4731 | 3.44 | 1.89 |
| Ridker PM et al. [61] | CORONA, 2007, multicenter | 76.4 | 73 | 2.7 | 5011 | 3.54 | 1.97 |
| Tavazzi L et al. [55] | GISSI-HF, 2008, centers in Italy | 77.4 | 68 | 3.9 | 4574 | 3.16 | 2.15 |
| Fellström BC et al. [62] | AURORA, 2009, multicenter | 62.1 | 64.1 | 3.2 | 2773 | 2.59 | 1.5 |
| Baigent C et al. [63] | SHARP, 2011, multicenter | 62.5 | 62 | 4.9 | 9270 | 2.77 | 1.69 |
| Cannon CP et al. [64] | IMPROVE-IT(p), 2015, multicenter | – | – | 6 | 17455 | 2.46 | 1.30 |
| Sabatine MS et al. [33] | FOURIER, 2017, multicenter | 75.4 | 62.5 | 2.2 | 27564 | 2.4 | 0.78 |
| Schwartz GG et al. [32] | ODYSSEY OUTCOMES, 2018, multicenter | 74.8 | 58 | 2.8 | 18924 | 2.25 | 0.97 |
Risk of bias assessment
There was unclear risk of bias in some of the items including allocation concealment, blinding of participants and personnel. All of the evaluated studies had a low risk of bias according to selective outcome reporting. None of the studies had an item with high risk of bias. Details of the quality of bias assessment are shown in Supplementary Table SIV.
Meta-analysis of the effect of lipid-lowering agents on all strokes
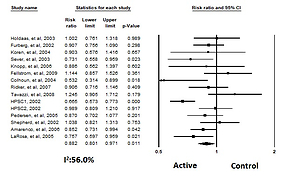
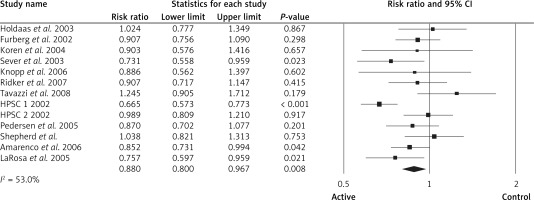
For those who achieved an LDL-C level ≤ 1.3 mmol/l (50 mg/dl), the RR of the effect of LLAs on all strokes was 0.81, 95% CI: 0.73–0.91, p < 0.001, ARR = 0.4%, NNT = 250, heterogeneity p = 0.273; I2 = 27%, n = 3 studies; TSA for this group indicates that the optimal sample size was reached, so a difference of 35% between groups is firmly discarded and no further studies are required. For those who achieved LDL-C between 1.3 mmol/l (50 mg/dl) and < 1.8 mmol/l (70 mg/dl) the RR of the effect of LLAs on all strokes was 0.83, 95% CI: 0.59–1.17, p = 0.299, ARR = 0.7%, NNT = 143, heterogeneity p = 0.025; I2 = 72%, n = 3 studies; TSA indicated that no boundaries were reached, implying that further studies are needed. For those at ≥ 1.8 mmol/l (70 mg/dl) the RR of the effect of LLAs on all strokes was 0.88, 95% CI: 0.80–0.96, p = 0.008, ARR = 0.7%, NNT = 143, heterogeneity p = 0.013; I2 = 53%, n = 13 studies, Figure 6). TSA indicated that both benefit boundaries and optimal sample size were reached and our conclusion is robust; the TSA finding was robust even with RRR = 20%.
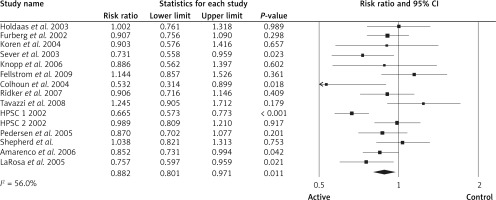
After grouping the patients according to those on statin therapy vs. non-statin therapy, for those on statin therapy the RR for all strokes was 0.88, 95% CI: 0.80–0.97, p = 0.011, ARR = 0.6%, NNT = 167, n = 15 studies, Figure 7), heterogeneity p = 0.004; I2 = 56%), TSA indicated that both benefit boundaries and optimal sample size were reached and our conclusion is robust (the TSA finding was robust even with RRR = 20%). For those on non-statin therapy the pooled estimate (RR) for all strokes was 0.81 (95% CI: 0.74–0.89, p < 0.001, ARR = 0.5%, NNT = 200, heterogeneity p = 0.431; I2 = 0.0%, n = 4 studies). TSA indicated that both benefit boundaries and optimal sample size were reached and our conclusion is robust (the TSA finding was robust even with RRR = 20%).
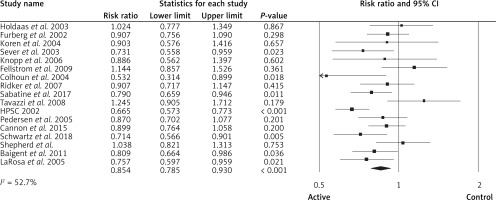
For those at primary prevention, the pooled estimate (RR) of the effect of lipid-lowering agents on all strokes was 0.85 (95% CI: 0.78–0.93, p < 0.001, ARR = 0.6%, NNT = 167, heterogeneity p = 0.006; I2 = 52.7%, n = 17 studies, Figure 8). TSA indicated that both benefit boundaries and optimal sample size were reached and our conclusion is robust (the TSA finding was robust even with RRR = 20%). For those at secondary prevention the pooled estimate (RR) of the effect of lipid-lowering agents on all strokes was 0.90 (95% CI: 0.79–1.01, p = 0.092, ARR = 1.2%, NNT = 83, heterogeneity p = 0.250; I2 = 24.5%, n = 2 studies). TSA for this group indicates that the optimal sample size was reached, so a difference of 35% between groups is firmly discarded and no further studies are required
Meta-analysis of the effect of lipid-lowering agents on ischemic strokes
For those who achieved LDL-C level ≤ 1.3 mmol/l, the pooled estimate (RR) of the effect of lipid-lowering agents on ischemic stroke was 0.78 (95% CI: 0.69–0.88, ARR = 0.5%, NNT = 200, heterogeneity p = 0.589; I2 = 0.0%, n = 3 studies), TSA indicated that the optimal sample size was reached and our conclusion is robust (the TSA finding was robust even with RRR = 20%). For those at 1.3 mmol/l (50 mg/dl) to 1.8 mmol/l (70 mg/dl) the pooled estimate (RR) of the effect of lipid-lowering agents on ischemic stroke was 0.76 (95% CI: 0.54–1.05, p = 0.104, ARR = 0.8%, NNT = 125, heterogeneity p = 0.099; I2 = 56.7%, n = 3 studies), TSA indicated that benefit boundaries were reached and our conclusion is robust. For those at ≥ 1.8 mmol/l (70 mg/dl), the pooled estimate (RR) of the effect of lipid-lowering agents on ischemic stroke was 0.75 (95% CI: 0.67–0.83, p < 0.001, ARR = 1.3%, NNT = 77, heterogeneity p = 0.423; I2 = 0.0%, n = 4 studies). TSA indicated that both benefit boundaries and optimal sample size were reached and our conclusion is robust (the TSA finding was robust even with RRR = 20%).
We divided people into statin therapy vs. non-statin therapy; for those on statin therapy the pooled estimate (RR) of ischemic stroke was 0.76 (95% CI: 0.69–0.84, p < 0.001, ARR = 1.3%, NNT = 77, heterogeneity p = 0.201; I2 = 32%, n = 6 studies). TSA indicated that both benefit boundaries and optimal sample size were reached and our conclusion is robust (the TSA finding was robust even with RRR = 20%). For those on non-statin therapy the pooled estimate (RR) of ischemic stroke was 0.77 (95% CI: 0.69–0.85, p < 0.001, ARR = 0.5%, NNT = 200, heterogeneity p = 0.694; I2 = 0.0%, n = 4 studies). TSA indicated that both benefit boundaries and optimal sample size were reached and our conclusion is robust (the TSA finding was robust even with RRR = 20%).
Meta-analysis of the effect of lipid-lowering agents on hemorrhagic strokes
For those who achieved LDL-C level ≤ 1.3 mmol/l (50 mg/dl), the pooled estimate (RR) of the effect of lipid-lowering agents on hemorrhagic stroke was 1.14 (95% CI: 0.84–1.54, heterogeneity p = 0.170; I2 = 43.6%, n = 3 studies), and TSA indicated that no boundaries were reached; thus further studies are needed. For those at 1.3 mmol/l (50 mg/dl) to 1.8 mmol/l (70 mg/dl) the pooled estimate (RR) of the effect of lipid-lowering agents on hemorrhagic stroke was 1.20 (95% CI: 0.84–1.69, p = 0.301, n = 2 studies), heterogeneity p = 0.960; I2 = 0.0%), and TSA indicated that no boundaries were reached; thus further studies are needed. For those at ≥ 1.8 mmol/l (70 mg/dl), the pooled estimate (RR) of the effect of lipid-lowering agents on hemorrhagic stroke was 1.25 (95% CI: 0.82–1.88 (p = 0.289), heterogeneity p = 0.050; I2 = 57.9%, n = 5 studies). TSA indicated that no boundaries were reached, so further studies are needed.
For those on statin therapy the pooled estimate (RR) of the effect of lipid-lowering agents on hemorrhagic stroke was 1.23 (95% CI: 0.88–1.71, p = 0.219, n = 6 studies), heterogeneity p = 0.090; I2 = 47.4%); TSA for this group indicates that the futility boundary was reached, so a difference of 35% between groups is firmly discarded and no further studies are required. For those on combination therapy the pooled estimate (RR) of the effect of lipid-lowering agents on hemorrhagic stroke 1.16 (95% CI: 0.90–1.48 (p = 0.232), heterogeneity p = 0.309; I2 = 16.5%, n = 4 studies). TSA for this group indicates that the futility boundary was reached, so a difference of 35% between groups is firmly discarded and no further studies are required.
Discussion
Our study sheds light on the debatable association between low LDL-C and different type of strokes. According to our results regarding the effect of LDL on the risk of stroke, there was a non-significant trend towards higher risk of hemorrhagic stroke for all investigated LDL-C levels (besides the highest LDL levels in cohort studies analyses), and higher LDL-C level (> 70 mg/dl) was associated with significantly higher risk of ischemic stroke. The analysis also confirmed a large effect of statin and non-statin lipid-lowering therapy on all strokes and ischemic strokes but without a significant effect on hemorrhagic strokes. This information can help to determine the optimal LDL-C range for stroke prevention and help to plan future studies with LLAs and different LDL-C targets. Further, well-designed studies are still needed to assess the effects of LLAs on hemorrhagic stroke. However, our data do not indicate any significant association between LDL-C thresholds and different lipid-lowering therapies and the risk of hemorrhagic stroke (only a numerical increase).
Hypercholesterolemia is associated with atherosclerosis due to the elevated concentration of oxidized or modified LDL-C leading to endothelial dysfunction [65]. Most of the epidemiological studies have reported an association between higher cholesterol level and an increased risk of ischemic stroke [8, 66–69]. On the other hand, given that cholesterol plays an important role in the structure formation of cell membranes, low cholesterol can be a potential risk factor for intracranial hemorrhage (ICH) [70]. It was found that a low level of cholesterol can lead to weakened endothelium, which results in arterial fragility and hemorrhage [70, 71]. It should be noted that weakened endothelium may be more susceptible to microaneurysms, which are one of the major pathological results of cerebral hemorrhage [71]. Some epidemiological studies demonstrated an inverse relationship between LDL-C levels and risk of ICH [72, 73], which was not definitely confirmed in our analysis. There might be other plausible mechanisms with regard to the potential association between low LDL-C level and increased risk of hemorrhagic stroke. Erythrocyte fragility can result from low cholesterol in erythrocyte membrane [74], LDL-related platelet activation and tissue factor expression [75] and impaired coagulation function [76]. Furthermore, microbleeds, as a risk factor for ICH, can be increased by low LDL-C concentration [77].
It is widely known that stroke is one of the major complications of cardiovascular disease (CVD). The association between LDL-C and each of these different types is likely to differ, as well as the link between different lipid-lowering therapies (statin vs. non-statin) and different types of stroke. Several prospective studies have evaluated the relationship between LDL-C and stroke, but the results are inconsistent. In a meta-analysis of data from 170,000 participants in 26 randomized trials, risk of hemorrhagic stroke was not increased by reduction in the LDL-C level with statins [78]; however, a non-significant increase in the incidence of hemorrhagic stroke by statins was reported [79]. Statin therapy for the secondary stroke prevention population was associated with higher risk of hemorrhagic stroke [79]. In addition, a meta-analysis of 23 randomized trials reported that each 1 mmol/l decrease in the achieved LDL-C level was associated with a 23.5% significant reduction in total stroke risk, whereas the risk of hemorrhagic stroke was not significantly increased by a lower achieved LDL-C level [80] – similar results are in fact observed also in our analyses.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial with 4731 participants after 4.9 years of follow-up showed that baseline LDL-C was not predictive for stroke. Only baseline HDL-C and LDL/HDL ratio were attributed with an outcome of ischemic stroke [81]. In accordance with this study, the Perindopril Protection Against Recurrent Stroke (PROGRESS) trial found no association between baseline LDL-C and risk of recurrent stroke [82]. In a cohort study of 23,367 participants with a mean follow-up of 7.5 ±2.9 years and 1031 strokes (814 ischemic, 77 hemorrhagic), LDL-C was significantly associated with increased risk of ischemic stroke by 8% [83]. In a meta-regression analysis from 49 trials including 312,175 participants (mean age: 62 years, 24% women) with approximately 4000 major vascular events during a mean of 4.3 years, lower achieved LDL-C level was significantly associated with lower rate of major coronary events (including coronary death and MI) for primary and secondary prevention trials [84]. Moreover, no significant association was reported between LDL-C and ischemic stroke in the Atherosclerosis Risk in Communities Study (ARIC) [7] and Framingham Study [85], and between LDL-C and hemorrhagic stroke [86, 87]. However, some limitations of these studies should be borne in mind, such as the relatively short follow-up (1–6 years) and small number of cases of hemorrhagic stroke. Moreover, in the Hisayama population-based study, with 2351 participants (271 strokes) followed up for 19 years, the authors reported no significant association between LDL-C concentration and stroke risk [15]. Most of the available clinical trials – including Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT), Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL), a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound Derived Coronary Atheroma Burden (ASTEROID), and Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) – reported that LDL-C lower than 70 mg/dl reversed the development of atherosclerotic plaque and reduced heart attack and all stroke rates [88–91]. In a meta-analysis of non-statin clinical trials, no significant positive association between LDL-C lowering and increased risk of hemorrhagic stroke was reported [24]. Finally, a nationwide cohort study of 52,421 hypercholesterolemic patients treated with open-labeled simvastatin for 6 years also reported no association between cerebral hemorrhage and serum lipid concentrations [92]. Our results are in line with the above-mentioned results, finding no significant association between LDL-C levels and the risk of stroke, although a non-significant positive trend was observed for this association, interestingly numerically the lowest for the LDL-C < 50 mg/dl (RR = 1.14) and the largest for LDL-C ≥ 70 mg/dl (RR = 1.25).
A prospective population-based cohort study with 9068 participants (age ≥ 55 years) during a mean follow-up of 9.7 years reported no association between LDL-C and intracerebral hemorrhage [93]. However, 36% of strokes were classified as unspecific because neuroimaging had not been performed, and the presence of residual confounding due to unmeasured determinants influenced the results of the study. A large multicenter, prospective population-based study with 5201 participants age 65 and older (mean follow-up: 7.5 years) reported a marginally significant association between LDL-C and ischemic stroke, and no association between LDL-C and hemorrhagic stroke. The authors demonstrated that lipid measure could not be important predicators of the outcomes of MI, ischemic stroke, hemorrhage stroke and total mortality [94]. The analysis of 21,798 participants of the European Prospective Investigation into Cancer in Norfolk (EPIC-NORFOLK) population study showed substantial heterogeneity in the association between the traditional atherosclerosis risk factors. The authors reported that LDL-C was a particularly strong risk factor for CAD, but it was less strongly associated with ischemic and hemorrhagic stroke [95]. They also reported that different associations between LDL and CAD vs. ischemic stroke were due to the morphological differences in plaques between intra- and extracranial arteries [96]. Interestingly, in a pooled cohort of the ARIC study (15,792 men and women, aged 45–64 years) and the Cardiovascular Heart Study (5888 men and women, aged 65 or over) the authors reported an inverse association between LDL-C and incident ICH [97]. Furthermore, a significant inverse association between LDL-C and hemorrhagic stroke was found in the Framingham Study [85]. Finally, a cohort study that included 96,043 participants (mean age: 51.3 years) with 9 years of follow-up reported that lower LDL-C concentration (LDL-C < 70 mg/dl) was significantly associated with higher risk of intracerebral hemorrhage, and this association became non-significant with LDL-C ≥ 70 mg/dl [51]. Moreover, a large population-based prospective study that included 91,219 participants (40–79 years of age, 30,802 men, 60,417 women) for 10.3 years showed a significant association between low LDL-C levels and increased risk of death due to intraparenchymal hemorrhage [73]. Similar results were observed in a pooled prospective cohort study that included 21,630 individuals with 135 cases of incident ICH, which showed that lower LDL-C was associated with higher risk of ICH [97]. Our results do not confirm any significant association between LDL levels (< and ≥ 70 mg/dl) and the risk of hemorrhagic stroke and significant reduction of ischemic strokes for all investigated achieved LDL-C levels.
We also found no significant effect of lipid-lowering agents regardless of the types (statin and non-statin therapy) and achieved level of LDL-C on the risk of hemorrhagic stroke. There are several studies available with regard to the effect of lipid-lowering agents on the risk of stroke. Statin therapy was effective in reducing CV events including stroke according to the degree of LDL-C level lowering [32, 33, 64]. With regard to the association between lowering LDL-C and relative CV risk reduction in statin and non-statin groups, a meta-regression study that included 312,175 individuals of 49 trials (mean age: 62 years, 20% women) reported that statin and non-statin therapies were associated with lower achieved LDL-C levels. Moreover, a significant linear association between achieved LDL-C and the rate of cardiovascular outcomes (including stroke) was found [84]. Similar to our findings, a prospective meta-analysis that included 90,056 individuals in 14 randomized trials of statin reported that risk of major coronary events, coronary revascularization and stroke was reduced by statin therapy by about one fifth per mmol/l in LDL cholesterol during 5-year follow-up [98]. The SPARCL trial reported that a lipid-lowering agent (atorvastatin 80 mg/day) significantly reduced the risk of recurrent stroke regardless of baseline LDL-C and other lipid parameters [99]. Similarly to our results, an insignificant association between statin therapy and the risk of hemorrhagic stroke was observed in the Cholesterol Treatment Trialists’ (CTT) meta-analysis of statin clinical trials [23]. The effectiveness of ezetimibe with simvastatin therapy in order to prevent stroke and other adverse cardiovascular events was assessed in the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), which was a multinational trial of 18,144 patients, of whom 641 (3.5%) experienced at least 1 stroke, and most were ischemic (n = 527 (82%)). They found a non-significant reduction in the first event of stroke of any type with ezetimibe plus simvastatin compared to simvastatin monotherapy. Ischemic stroke was significantly reduced by adding ezetimibe to simvastatin. However, no significant increase in hemorrhagic stroke was observed in statin therapy with ezetimibe [100]. A meta-analysis involving data from the analysis of 4405 patients who completed phase 2 or 3 studies of evolocumab during 1 year, and a randomized trial on alirocumab including 2341 patients during 1.5 years follow-up reported no significant effect of PCSK9 inhibition on stroke rate. The number of ischemic strokes was small in both trials, and no hemorrhagic stroke was reported in either study [101]. Moreover, an animal study showed that PCSK9-/- mice had lower LDL-C, high-density lipoprotein cholesterol, and total cholesterol levels that PCSK9+/+ mice before and after 1 month on the high-fat/high-cholesterol diet. They found that hemorrhagic complications after acute ischemic stroke should not be increased by PCSK9 inhibition which leads to LDL-C lowering [102, 103]. The subject is still debatable, and the most recent results also give opposite results for extremely and very low LDL-C and the risk of hemorrhagic stroke and the role of lipid-lowering therapies, mainly statins, in this association [104, 105]. Based on the available results it seems that we should consider higher LDL-C levels for patients with the risk of hemorrhagic stroke (70–100 mg/dl) with the consideration of using non-statin drugs for these patients (PCSK9 inhibitors) [104–111].
Our study has some limitations. We observed a relatively high rate of heterogeneity with regard to the link between LDL concentration and stroke; thus, to obtain more reliable conclusions, more studies with higher resolution are needed. We had sufficient statistical power and a low level of type I and II error for the RCTs with regard to the total stroke and ischemic risk, but not for the hemorrhagic stroke, to obtain reliable results. Cohort studies were adjusted for the varied range of the co-variants, which might have led to the high level of the heterogeneity between studies. We performed TSA to evaluate and decrease the chance of type I and II error, which was a strength of the study.
In conclusion, our study sheds light on the debatable association between low LDL-C and different types of strokes. LDL-C reduction with available therapies is in general associated with the reduction of all strokes and ischemic strokes; such an association was not seen for hemorrhagic stroke, where no significant results were observed. This information can help determine the optimal LDL-C range for stroke prevention and help plan future LLA studies. Further studies are still needed to determine the effects of low to extremely low LDL-C levels on hemorrhagic stroke and the role of LLAs.