Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
HEPATOLOGY / STATE OF THE ART PAPER
Recent advances in immune checkpoint inhibitor-based therapy of advanced hepatocellular carcinoma
1
Department of Oncology, Guangxi International Zhuang Medicine Hospital, Guangxi University of Chinese Medicine, Nanning, China
2
Department of Oncology, First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning, China
These authors had equal contribution to this work
Submission date: 2024-01-09
Final revision date: 2024-04-20
Acceptance date: 2024-05-15
Online publication date: 2024-06-07
Corresponding author
Guodong Huang
Guangxi International Zhuang Medicine Hospital Affiliated to Guangxi University of Chinese Medicine 8 Qiuyue Road Nanning 530201l, China Phone/fax: 867713376615
Guangxi International Zhuang Medicine Hospital Affiliated to Guangxi University of Chinese Medicine 8 Qiuyue Road Nanning 530201l, China Phone/fax: 867713376615
KEYWORDS
immune checkpoint inhibitorimmunotherapycancerprognostic biomarkertherapeutic responsesurgical resection
TOPICS
ABSTRACT
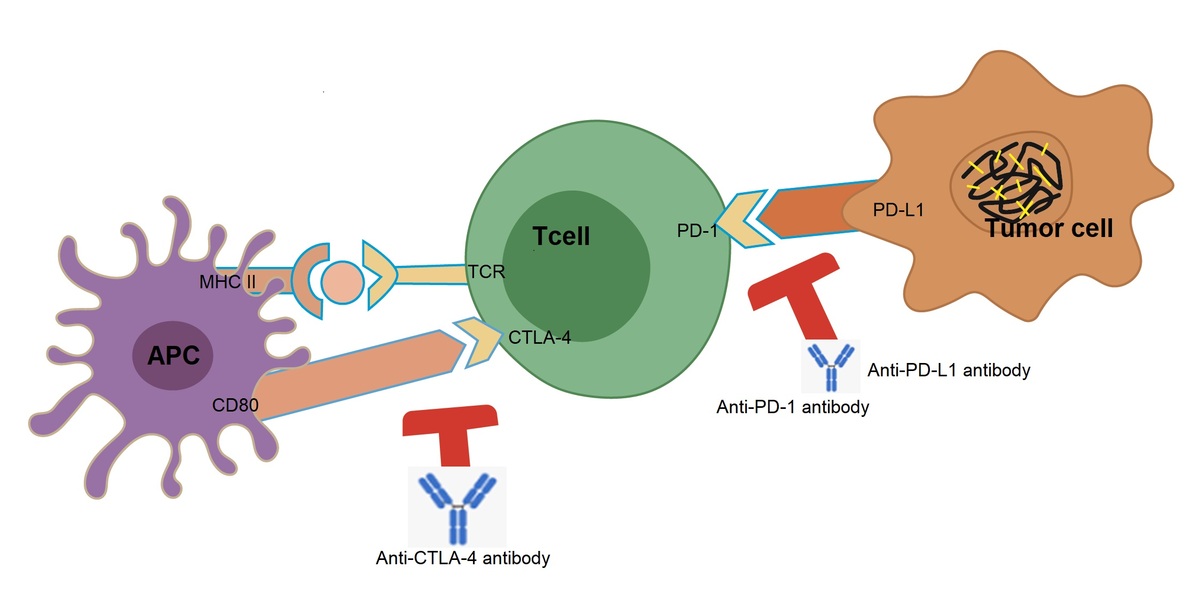
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and is associated with a high mortality rate. Its occult origin often results in the loss of the optimal timeframe for liver transplantation and resection. During the past few decades, tremendous advances in the treatment of HCC have been achieved, and immunotherapy has become an attractive approach with promising results in clinical trials. In the present work, we review immune checkpoint inhibitors (ICIs) for their function and role in treating cancers, particularly advanced HCC, summarize recent therapeutic progress with various ICIs or their combinations with other options/therapeutic agents, and discuss works related to the development of biomarkers that predict therapeutic response as well as the limitations of ICIs. Future directions for immune checkpoint (ICP) therapy are also addressed.
REFERENCES (140)
1.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021; 7: 6.
2.
Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol 2017; 34: 153-9.
3.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7-30.
4.
Marrero CR, Marrero JA. Viral hepatitis and hepatocellular carcinoma. Arch Med Res 2007; 38: 612-20.
5.
Heller S, Valencia-Mayoral P. Treatment of viral hepatitis in children. Arch Med Res 2007; 38: 702-10.
6.
Shi F, Xiao F, Ding P, et al. Long noncoding RNA highly up-regulated in liver cancer predicts unfavorable outcome and regulates metastasis by MMPs in triple-negative breast cancer. Arch Med Res 2016; 47: 446-53.
7.
Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int 2019; 13: 125-37.
8.
Schlachterman A, Craft WW Jr, Hilgenfeldt E, et al. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol 2015; 21: 8478-91.
9.
Tsurusaki M, Murakami T. Surgical and locoregional therapy of HCC: TACE. Liver Cancer 2015; 4: 165-75.
10.
Shim SJ, Seong J, Han KH, et al. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int 2005; 25: 1189-96.
11.
Brunetti O, Gnoni A, Licchetta A, et al. Predictive and prognostic factors in HCC patients treated with sorafenib. Medicina 2019; 55: 707.
12.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378-90.
13.
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25-34.
14.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163-73.
15.
Kuzuya T, Ishigami M, Ito T, et al. Sorafenib vs. lenvatinib as first-line therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Anticancer Res 2020; 40: 2283-90.
16.
Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56-66.
17.
Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015; 16: 859-70.
18.
Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022; 19: 151-72.
19.
Lin Z, Li J, Zhang J, et al. Metabolic reprogramming driven by IGF2BP3 promotes acquired resistance to EGFR inhibitors in non-small cell lung cancer. Cancer Res 2023; 83: 2187-207.
20.
Liu Y, Cao X. Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med 2016; 94: 509-22.
21.
Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res 2015; 21: 687-92.
22.
Ikeda M, Morizane C, Ueno M, et al. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol 2018; 48: 103-14.
23.
Kuol N, Stojanovska L, Nurgali K, et al. PD-1/PD-L1 in disease. Immunotherapy 2018; 10: 149-60.
24.
Puza CJ, Bressler ES, Terando AM, et al. The emerging role of surgery for patients with advanced melanoma treated with immunotherapy. J Surg Res 2019; 236: 209-15.
25.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350-5.
26.
Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016; 375: 1845-55.
27.
Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer 2019; 119: 1-10.
28.
Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013; 19: 5300-9.
29.
Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16: 522-30.
30.
Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges 2011; 9: 277-86.
31.
Cheng H, Sun G, Chen H, et al. Trends in the treatment of advanced hepatocellular carcinoma: immune checkpoint blockade immunotherapy and related combination therapies. Am J Cancer Res 2019; 9: 1536-45.
32.
van Doorn DJ, Takkenberg RB, Klumpen HJ. Immune checkpoint inhibitors in hepatocellular carcinoma: an overview. Pharmaceuticals (Basel) 2020; 14: 3.
33.
Yang K, Li J, Sun Z, et al. Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review. Ther Adv Med Oncol 2020; 12: 1758835920975353.
34.
Lin CL, Kao JH. Risk stratification for hepatitis B virus related hepatocellular carcinoma. J Gastroenterol Hepatol 2013; 28: 10-7.
35.
Doherty DG. Immunity, tolerance and autoimmunity in the liver: a comprehensive review. J Autoimmun 2016; 66: 60-75.
36.
Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol 2017; doi: 10.1016/j.jhep.2017.09.007.
38.
Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology 2017; 153: 1107-19 e10.
39.
Noguchi T, Ward JP, Gubin MM, et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res 2017; 5: 106-17.
40.
Chen X, Du Y, Hu Q, et al. Tumor-derived CD4+CD25+regulatory T cells inhibit dendritic cells function by CTLA-4. Pathol Res Pract 2017; 213: 245-9.
41.
Nagai K. Co-inhibitory receptor signaling in T-cell-mediated autoimmune glomerulonephritis. Front Med 2020; 7: 584382.
42.
Kortekaas KE, Santegoets SJ, Sturm G, et al. CD39 identifies the CD4(+) tumor-specific T-cell population in human cancer. Cancer Immunol Res 2020; 8: 1311-21.
43.
Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005; 25: 9543-53.
44.
Baumeister SH, Freeman GJ, Dranoff G, et al. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol 2016; 34: 539-73.
45.
Shrestha R, Prithviraj P, Anaka M, et al. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front Oncol 2018; 8: 269.
46.
Larkins E, Blumenthal GM, Yuan W, et al. FDA Approval Summary: pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy. Oncologist 2017; 22: 873-8.
47.
Mendes D, Rigueiro G, Silva RS, et al. Intensive safety monitoring program of antineoplastic medicines: a pilot study in a Portuguese oncology hospital. J Oncol Pharm Pract 2020; 26: 133-40.
48.
Haitsma G, Patel H, Gurumurthy P, et al. Access to anti-cancer drugs in India: is there a need to revise reimbursement policies? Expert Rev Pharmacoecon Outcomes Res 2018; 18: 289-96.
49.
El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492-502.
50.
Lo B, Fritz JM, Su HC, et al. CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood 2016; 128: 1037-42.
51.
Delyon J, Mateus C, Lefeuvre D, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol 2013; 24: 1697-703.
52.
Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59: 81-8.
53.
Sangro B, Yau T, El-Khoueiry AB, et al. Exposure-response analysis for nivolumab plus ipilimumab combination therapy in patients with advanced hepatocellular carcinoma (CheckMate 040). Clin Transl Sci 2023; 16: 1445-57.
54.
Sun T, Zhang W, Li Y, et al. Combination immunotherapy with cytotoxic T-lymphocyte-associated antigen-4 and programmed death protein-1 inhibitors prevents postoperative breast tumor recurrence and metastasis. Mol Cancer Ther 2020; 19: 802-11.
55.
Wei SC, Levine JH, Cogdill AP, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 2017; 170: 1120-33 e17.
56.
Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377: 1345-56.
57.
Butow PN, Turner J, Gilchrist J, et al. Randomized trial of ConquerFear: a novel, theoretically based psychosocial intervention for fear of cancer recurrence. J Clin Oncol 2017; 35: 4066-77.
58.
Rini BI, Signoretti S, Choueiri TK, et al. Long-term outcomes with nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. J Immunother Cancer 2022; 10: e005445.
59.
Datta M, Coussens LM, Nishikawa H, et al. Reprogramming the tumor microenvironment to improve immunotherapy: emerging strategies and combination therapies. Am Soc Clin Oncol Educ Book 2019; 39: 165-74.
60.
Shigeta K, Datta M, Hato T, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 2020; 71: 1247-61.
61.
Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022; 76: 862-73.
62.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma N Engl J Med 2020; 382: 1894-905.
63.
Zhang W, Bi X, Sun Y, et al. Preliminary results of sintilimab plus different dose of IBI305 (anti-VEGF monoclonal antibody) in patients with advanced hepatocellular carcinoma: a phase Ib study. J Clin Oncol 2020; 38: 3079-80.
64.
Kudo M, Motomura K, Wada Y, et al. Avelumab in combination with axitinib as first-line treatment in patients with advanced hepatocellular carcinoma: results from the phase 1b VEGF Liver 100 Trial. Liver Cancer 2021; 10: 249-59.
65.
Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020; 38: 2960-70.
66.
Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29: 482-91.
67.
Qin S, Chen Z, Liu Y, et al. A phase II study of anti–PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J Clin Oncol 2019; 37: 4074-5.
68.
Chiang CL, Chan ACY, Chiu KWH, et al. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol 2019; 9: 1157.
69.
Chew V, Lee YH, Pan L, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019; 68: 335-46.
70.
Yu JI, Lee SJ, Lee J, et al. Clinical significance of radiotherapy before and/or during nivolumab treatment in hepatocellular carcinoma. Cancer Med 2019; 8: 6986-94.
71.
Pinato DJ, Cole T, Bensch B, Tait P. 750PA phase Ib study of pembrolizumab following trans-arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): PETAL. Ann Oncol 2019; 30 (suppl. 5).
72.
Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021; 18: 293-313.
73.
Cheung TT, Ma KW, She WH. A review on radiofrequency, microwave and high-intensity focused ultrasound ablations for hepatocellular carcinoma with cirrhosis. Hepatobiliary Surg Nutr 2021; 10: 193-209.
74.
Inarrairaegui M, Sangro B. Selective internal radiation therapy approval for early HCC: what comes next? Hepatology 2021; 74: 2333-5.
75.
Matsuo Y. Stereotactic body radiotherapy for hepatocellular carcinoma: a brief overview. Curr Oncol 2023; 30: 2493-500.
76.
Cardarelli-Leite L, Hadjivassiliou A, Klass D, et al. Current locoregional therapies and treatment strategies in hepatocellular carcinoma. Curr Oncol 2020; 27 (Suppl 3): S144-51.
77.
Kaseb A, Cao H, Abugaba Y. Final results of a randomized, open label, perioperative phase II study evaluating nivolumab alone or nivolumab plus ipilimumab in patients with resectable HCC. J Clin Oncol 2020; 38: 4599-600.
78.
Shi XJ, Jin X, Wang MQ, et al. Effect of resection following downstaging of unresectable hepatocellular carcinoma by transcatheter arterial chemoembolization. Chin Med J 2012; 125: 197-202.
79.
Zhu XD, Huang C, Shen YH, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer 2021; 10: 320-9.
80.
Cui H, Dai G, Guan J. Programmed cell death protein-1 (PD-1)-targeted immunotherapy for advanced hepatocellular carcinoma in real world. Onco Targets Ther 2020; 13: 143-9.
81.
Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther 2019; 49: 1323-33.
82.
Ahn BC, Pyo KH, Xin CF, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol 2019; 145: 1613-23.
83.
Khozin S, Carson KR, Zhi J, et al. Real-world outcomes of patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors in the year following U.S. Regulatory Approval. Oncologist 2019; 24: 648-56.
84.
Furuya N, Nishino M, Wakuda K, et al. Real-world efficacy of atezolizumab in non-small cell lung cancer: a multicenter cohort study focused on performance status and retreatment after failure of anti-PD-1 antibody. Thorac Cancer 2021; 12: 613-8.
85.
Khozin S, Miksad RA, Adami J, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer 2019; 125: 4019-32.
86.
Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer 2019; 7: 193.
87.
Juneja VR, McGuire KA, Manguso RT, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 2017; 214: 895-904.
88.
Qayyum A, Hwang KP, Stafford J, et al. Immunotherapy response evaluation with magnetic resonance elastography (MRE) in advanced HCC. J Immunother Cancer 2019; 7: 329.
89.
Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 2018; 19: 737-46.
90.
Kugel CH 3rd, Douglass SM, Webster MR, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res 2018; 24: 5347-56.
91.
Feun LG, Li YY, Wu C, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer 2019; 125: 3603-14.
92.
Macek Jilkova Z, Aspord C, Kurma K, et al. Immunologic features of patients with advanced hepatocellular carcinoma before and during sorafenib or anti-programmed death-1/programmed death-L1 treatment. Clin Transl Gastroenterol 2019; 10: e00058.
93.
Kim HD, Song GW, Park S, et al. Association between expression level of PD1 by tumor-infiltrating CD8(+) T cells and features of hepatocellular carcinoma. Gastroenterology 2018; 155: 1936-50 e17.
94.
Pfister D, Nunez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021; 592: 450-6.
95.
Cassidy FH, Yokoo T, Aganovic L, et al. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics 2009; 29: 231-60.
96.
Murai H, Kodama T, Maesaka K, et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology 2023; 77: 77-91.
97.
Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res 2019; 25: 989-99.
98.
Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 2018; 4:1543-52
99.
Chen Y, Li X, Liu G, et al. ctDNA concentration, MIKI67 mutations and hyper-progressive disease related gene mutations are prognostic markers for camrelizumab and apatinib combined multiline treatment in advanced NSCLC. Front Oncol 2020; 10: 1706.
100.
Lu Z, Zou J, Hu Y, et al. Serological markers associated with response to immune checkpoint blockade in metastatic gastrointestinal tract cancer. JAMA Netw Open 2019; 2: e197621.
101.
Zhang L, Wu L, Chen Q, et al. Predicting hyperprogressive disease in patients with advanced hepatocellular carcinoma treated with anti-programmed cell death 1 therapy. EClinicalMedicine 2021; 31: 100673.
102.
Choi WM, Kim JY, Choi J, et al. Kinetics of the neutrophil-lymphocyte ratio during PD-1 inhibition as a prognostic factor in advanced hepatocellular carcinoma. Liver Int 2021; 41: 2189-99.
103.
Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23: 4242-50.
104.
Wu L, Quan W, Luo Q, et al. Identification of an immune-related prognostic predictor in hepatocellular carcinoma. Front Mol Biosci 2020; 7: 567950.
105.
Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22: 4585-93.
106.
Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 2016; 165: 35-44.
107.
Lee V, Le DT. Efficacy of PD-1 blockade in tumors with MMR deficiency. Immunotherapy 2016; 8: 1-3.
108.
Zhao P, Li L, Jiang X, et al. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol 2019; 12: 54.
109.
Kamada T, Togashi Y, Tay C, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA 2019; 116: 9999-10008.
110.
Kallies A, Zehn D, Utzschneider DT. Precursor exhausted T cells: key to successful immunotherapy? Nat Rev Immunol 2020; 20: 128-36.
111.
Meyer C, Cagnon L, Costa-Nunes CM, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 2014; 63: 247-57.
112.
Gao Y, Yang J, Cai Y, et al. IFN-gamma-mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K-AKT signaling. Int J Cancer 2018; 143: 931-43.
113.
Sasaki A, Nakamura Y, Mishima S, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer 2019; 22: 793-802.
114.
Presti D, Dall’Olio FG, Besse B, et al. Tumor infiltrating lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: a systematic review. Crit Rev Oncol Hematol 2022; 177: 103773.
115.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348: 56-61.
116.
Shi Y, Lei Y, Liu L, et al. Integration of comprehensive genomic profiling, tumor mutational burden, and PD-L1 expression to identify novel biomarkers of immunotherapy in non-small cell lung cancer. Cancer Med 2021; 10: 2216-31.
117.
Zhang X, Wang Y, Gari A, et al. Pan-cancer analysis of PARP1 alterations as biomarkers in the prediction of immunotherapeutic effects and the association of its expression levels and immunotherapy signatures. Front Immunol 2021; 12: 721030.
118.
Schwartz DJ, Rebeck ON, Dantas G. Complex interactions between the microbiome and cancer immune therapy. Crit Rev Clin Lab Sci 2019; 56: 567-85.
119.
Zou XL, Li XB, Ke H, et al. Prognostic value of neoantigen load in immune checkpoint inhibitor therapy for cancer. Front Immunol 2021; 12: 689076.
120.
Fang W, Wu CH, Sun QL, et al. Novel tumor-specific antigens for immunotherapy identified from multi-omics profiling in thymic carcinomas. Front Immunol 2021; 12: 748820.
121.
Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014; 11: 473-81.
122.
Hata A, Katakami N, Yoshioka H, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: comparison between T790M mutation-positive and mutation-negative populations. Cancer 2013; 119: 4325-32.
123.
Denis MG, Vallee A, Theoleyre S. EGFR T790M resistance mutation in non small-cell lung carcinoma. Clin Chim Acta 2015; 444: 81-5.
124.
Kuiper JL, Heideman DA, Thunnissen E, et al. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer 2014; 85: 19-24.
125.
Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008; 27: 4702-11.
126.
Watanabe S, Yoshida T, Kawakami H, et al. T790M-selective EGFR-TKI combined with dasatinib as an optimal strategy for overcoming EGFR-TKI resistance in T790M-positive non-small cell lung cancer. Mol Cancer Ther 2017; 16: 2563-71.
127.
Kwak Y, Kim SI, Park CK, et al. C-MET overexpression and amplification in gliomas. Int J Clin Exp Pathol 2015; 8: 14932-8.
128.
Presutti D, Santini S, Cardinali B, et al. MET gene amplification and MET receptor activation are not sufficient to predict efficacy of combined MET and EGFR inhibitors in EGFR TKI-resistant NSCLC cells. PLoS One 2015; 10: e0143333.
129.
Watermann I, Schmitt B, Stellmacher F, et al. Improved diagnostics targeting c-MET in non-small cell lung cancer: expression, amplification and activation? Diagn Pathol 2015; 10: 130.
130.
Janbabai G, Oladi Z, Farazmandfar T, et al. The prognostic impact of EGFR, ErbB2 and MET gene amplification in human gastric carcinomas as measured by quantitative real-time PCR. J Cancer Res Clin Oncol 2015; 141: 1945-52.
131.
Xiang H, Bender BC, Reyes AE 2nd, et al. Onartuzumab (MetMAb): using nonclinical pharmacokinetic and concentration-effect data to support clinical development. Clin Cancer Res 2013; 19: 5068-78.
132.
Milella M, Falcone I, Conciatori F, et al. PTEN: multiple functions in human malignant tumors. Front Oncol 2015; 5: 24.
133.
Hagiwara S, Kudo M, Nagai T, et al. Activation of JNK and high expression level of CD133 predict a poor response to sorafenib in hepatocellular carcinoma. Br J Cancer 2012; 106: 1997-2003.
134.
Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 2007; 132: 2542-56.
135.
Haga Y, Kanda T, Nakamura M, et al. Overexpression of c-Jun contributes to sorafenib resistance in human hepatoma cell lines. PLoS One 2017; 12: e0174153.
136.
Sasaki R, Kanda T, Fujisawa M, et al. Different mechanisms of action of regorafenib and lenvatinib on Toll-like receptor-signaling pathways in human hepatoma cell lines. Int J Mol Sci 2020; 21: 3349.
137.
Li DD, Zhang YF, Xu HX, et al. The role of BRAF in the pathogenesis of thyroid carcinoma. Front Biosci (Landmark Ed) 2015; 20: 1068-78.
138.
Nguyen-Ngoc T, Bouchaab H, Adjei AA, et al. BRAF alterations as therapeutic targets in non-small-cell lung cancer. J Thorac Oncol 2015; 10: 1396-403.
139.
Sheppard K, Kinross KM, Solomon B, et al. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog 2012; 17: 69-95.
140.
van Cruijsen H, van der Veldt A, Hoekman K. Tyrosine kinase inhibitors of VEGF receptors: clinical issues and remaining questions. Front Biosci 2009; 14: 2248-68.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.