Introduction
Psoriatic arthritis (PsA) is a debilitating seronegative arthropathy with many different clinical and radiographic manifestations [1]. Psoriatic arthritis is typically diagnosed in people aged 40 to 50 years, but it also occurs in younger adults and children [2]. Psoriatic arthritis affects 7% to 48% of patients with psoriasis [2, 3]. Usually, PsA follows the onset of psoriasis, but sometimes it develops before psoriatic skin lesions [2]. Notably, patients with psoriasis and without PsA may have subclinical bone changes, such as bone formation in joint entheses [4].
Psoriatic arthritis is diagnosed according to the Classification Criteria for Psoriatic Arthritis (CASPAR) [5]. In Europe and North America, the diagnosis of PsA is missed in more than one-third of patients with psoriasis [6]. Physicians should diagnose PsA early because delaying diagnosis, even by 6 months, increases the risks of joint erosions and poor long-term physical function [7]. Early diagnosis enables prompt treatment initiation, which improves clinical outcomes and reduces both disease severity and joint damage [5]. To improve the detection of PsA, one can evaluate the four following symptoms: peripheral inflammatory pain, axial inflammatory pain, dactylitis, and buttock and sciatic pain. In particular, one might focus on those patients with psoriasis with nail dystrophy, scalp involvement, extensive skin involvement, and intergluteal or perianal lesions [5].
Symptoms of PsA are heterogeneous [8]. Table I presents different clinical subtypes of PsA, including enthesitis [8–15]. In up to 60% of patients, PsA begins with oligoarthritis (≤ 4 joints), but it may affect more joints as the disease progresses (polyarthritis, ≥ 5 joints) [10]. Dactylitis is characteristic of PsA; it affects feet more often than hands (65% vs. 24%). Only 12% of patients with PsA have dactylitis both in feet and hands [13]. Dactylitis carries a risk of more aggressive disease in the affected fingers [14]. Patients with PsA often have inflamed entheses (enthesitis) [10]. Evidence supports the hypothesis that PsA-associated inflammation begins in entheses [15], which are exposed not only to biochemical stress, but also to repeated micro-trauma [4]. In genetically predisposed individuals, micro-trauma to entheses may lead to inflammation [1], which then spreads to surrounding structures, particularly to the nail matrix and synovium [1, 15].
Table I
Clinical examination is crucial to diagnose PsA. On clinical examination, joints affected by PsA are usually painful and tender, but swelling does not always occur. Moreover, patients often have morning stiffness [9, 10]. In addition to clinical examination, new imaging techniques can now be used in daily practice to improve the diagnosis of PsA (Table II) [8, 9, 14, 16].
Because patients with PsA have heterogeneous symptoms, they often need the care of many specialists. When joint inflammation precedes psoriasis, PsA might be difficult to diagnose. Imaging and laboratory studies can help rule out rheumatoid arthritis, spondyloarthritis, osteoarthritis, and reactive arthritis [17–19]. Analysis of synovial fluid can help diagnose gout, chondrocalcinosis, and septic arthritis [17–19].
Patients with psoriasis often seek assistance in orthopedic clinics. Among patients with PsA, Haque et al. reported that 36% of orthopedic operations and ~10% of diagnostic arthroscopies were done before the diagnosis of PsA [20]. Moreover, the involvement of large joints, such as the hip and knee, impairs activities of daily living in patients with PsA. To maintain daily function, patients need individualized therapy, which may include surgical management [20].
Here, we review current data regarding orthopedic management of diseases of the lower extremities in patients with PsA.
Orthopedic interventions in PsA
At first, PsA was considered to be a less severe disease than rheumatoid arthritis, but we know now that PsA is more aggressive than initially thought. Structural damage in PsA includes not only bone erosions and cartilage loss but also new bone formation and joint remodeling. Severe osteolysis leads to the development of arthritis mutilans [20, 21]. In most patients, PsA is treated pharmacologically with non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), and biologic agents [22]. In PsA, biologic therapy improves the control of inflammation, inhibits both the formation of joint erosions and joint destruction, and improves joint function [8, 21]. When pharmacological treatment is insufficient, patients with degenerative joint changes may benefit from surgical treatment, which can improve quality of life and restore or maintain joint function. However, surgery does not change the natural course of PsA. Patients receiving DMARDs alone or combined with tumor necrosis factor α (TNF-α) inhibitors may need orthopedic intervention at some stage of the disease [20].
In PsA, joint destruction involves cartilage, ligaments, synovium, and bones [8, 20]. Because cartilage has a very limited healing ability, surgeons should preserve hyaline cartilage [23]. Operations preserving joints aim to restore normal joint axis and joint stability. They involve correction of both intra-articular and extra-articular abnormalities. Total joint arthroplasty is the best treatment for end-stage arthritis in the hip and knee joints; it improves the range of motion and reduces joint pain. Due to limited evidence, we do not know what surgery to choose in particular patients with PsA (Table III) [20, 24–35]. Qualifying patients with PsA for surgery needs an individualized approach and the involvement of dermatologists, rheumatologists, and orthopedic surgeons. Joints that are most painful or most functionally important should be corrected first. Adequate surgical correction of lower extremity joints can restore ambulation and improve pain in patients with PsA.
Table III
Orthopedic procedures in lower extremities in patients with PsA or psoriasis, including indications, likely outcomes, and potential complications [20, 24–33]
| Procedure | Indications | Benefits | Potential complications |
|---|---|---|---|
| Synovectomy [20, 24, 25] | Synovitis, persistent joint effusion | Removal of inflammatory synovium, decrease in proinflammatory cytokines, and effusion control | Side effects were not reported [20, 24, 25] Potential complications include infection, hematoma, and joint contractures [26] |
| Osteotomy [27] | Joint malalignment | Restoration of mechanical properties of joints | Complications were not reported in patients with psoriatic arthritis [27] Potential complications include infection, hematoma, persistent pain, over- and undercorrection [26] |
| Debridement [28] | Loose bodies, degenerative changes of labrum, menisci, or articular cartilage | Decrease in proinflammatory cytokines | Complications were not reported in patients with psoriatic arthritis [28]. Potential complications include infection, hematoma, iatrogenic joint destruction [26] |
| Tissue reconstruction [20] (ligaments, menisci, labrum, cartilage) | Ligament insufficiency, chondral defects, labral tears, meniscus tears | Restoration of mechanical properties of joints | Complications were not reported in patients with psoriatic arthritis [20]. Potential complications include infection, hematoma, iatrogenic joint destruction |
| Arthrodesis [20, 25, 29] | Painful end-stage arthrosis in patients with contraindications to arthroplasty | Painless weight-bearing joints | The following have been reported: delayed bone union, nonunion of arthrodesis, Koebner phenomenon [25, 28]. Potential surgical side effects include loss of motion in adjacent joints, infection, malalignment, hematoma, persistent pain, deep venous thrombosis [26] |
| Arthroplasty [29–33] | Painful end-stage arthrosis | Restoration of painless range of motion in joints | Reported complications [32, 33] include infection, pulmonary embolism, paralytic ileus, deep venous thrombosis, exacerbation of psoriasis (similar risk as in the general population). Potential complications include periprosthetic fractures, hematoma, implant failure, osteolysis, dislocation [26] |
When planning surgery in patients with PsA, one must remember that injury and stress related to the procedure may lead to a psoriatic outbreak or exacerbations [8, 22, 33]. There are limited data about the Koebner phenomenon after surgery [8, 22, 33]. Postoperative infections can be avoided with adequate skin preparation before surgery [35]. Because post-operative sepsis after Charnley low-friction arthroplasty was more frequent in patients with psoriasis (n = 38) than in patients with osteoarthritis, prophylactic antibiotic therapy was recommended [32]. It is recommended not to operate through psoriatic plaques [8]. Postoperative infections and impaired wound-healing are a concern in patients who receive biologic agents, methotrexate, or cyclosporine. Recommendations for perioperative adjustment of pharmacological treatment in patients with PsA are listed in Table IV [36–39].
Table IV
Recommendations for medication adjustment before orthopedic surgery in patients with PsA
| Study | Medication | |||
|---|---|---|---|---|
| Smith et al. [36] | Discontinuation of biologic therapy prior to surgery | |||
| Infliximab 4–6 weeks | Adalimumab 6–8 weeks | Etanercept 2 weeks | Ustekinumab 12 weeks | |
| Therapy may be restarted postoperatively when there is no evidence of infection and wound healing is satisfactory | ||||
| Ohtsuki et al. [37] | Surgery feasible after a necessary time from last dose | |||
| Infliximab 4 weeks | Adalimumab 2 weeks | Etanercept no data | Ustekinumab 8 weeks | |
| Therapy may be restarted postoperatively when there is no evidence of infection and the wound has healed | ||||
| Ossorio et al. [38] | Discontinuation of biologic therapy prior to surgery | |||
| Infliximab 6 weeks | Adalimumab 2 weeks | Etanercept 1 week | Golimumab/certolizumab/tocilizumab 4 weeks | |
| Therapy may be restarted postoperatively when there is no evidence of infection and the wound has healed | ||||
| Cyclosporine A should be stopped one week before orthopedic surgery and reintroduced 2 weeks after the surgery | ||||
| Sreekumar et al. [39] | Methotrexate should not be stopped before elective orthopedic surgery in patients with disease controlled on methotrexate before surgery | |||
Hip
Psoriatic hip arthropathy is uncommon, but it is associated with an early onset of PsA [29]. Patients often have bilateral hip arthropathy and, due to rapid joint destruction, need hip arthroplasty at a young age [29, 39]. Haque et al. [20] and Michet et al. [29] observed that the mean duration of PsA at the time of arthroplasty was about 5–5.5 years. However, Zangger et al. reported that the need for surgical intervention increases with disease progression [25]. Total hip arthroplasty (THA) is the most common joint-sacrificing procedure in PsA, and it is recommended in patients with end-stage arthritis and significant disability [20, 25, 27]. Total hip arthroplasty reduces pain and improves joint function (Figure 1 A, B) [8].
Figure 1
A – Preoperative radiograph of a 65-year-old woman admitted to the hospital for total hip replacement shows severe joint space narrowing (white arrow), bone erosions (white star), and migration of femoral head. There are no osteophytes, which are characteristic of osteoarthritis. B – Radiograph obtained after total hip arthroplasty
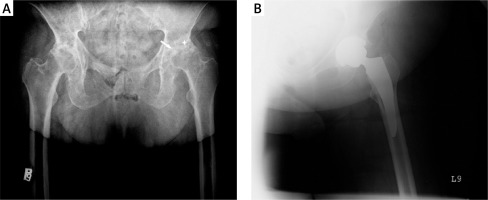
Among patients with PsA, indications for THA include severe joint damage assessed with the Steinbrocker grading system, a higher number of actively involved joints, and severe pain at rest or when walking [8, 25, 30]. Total hip arthroplasty is performed most commonly in patients with polyarticular PsA, but also in patients with oligoarticular PsA [20, 27] or spondylitis predominant PsA [20, 29].
In patients with PsA, joint pain might be caused by conditions other than PsA, particularly by osteoarthritis (OA). Osteoarthritis coexists with psoriasis, particularly in patients aged > 70 years, and these patients require specific treatment protocols [31, 32, 40]. Mandl et al. analyzed data of patients who underwent THA, including 63 patients with PsA, 153 patients with psoriasis, and 915 patients with OA who served as controls [31]. There were no differences in preoperative and postoperative WOMAC pain scores (Western Ontario and McMaster Universities OA Index) between the three groups. Thus, it seems that neither PsA nor psoriasis are independent risk factors for poor postoperative function or joint pain after THA [21]. In contrast, previous studies reported that postoperative outcomes after THA were worse in patients with PsA than in other patients [27, 40]. Hip surgery is performed increasingly often in young patients, which could be due to improper qualification of patients. Patients with polyarticular PsA or with spondylitis-predominant PsA should be assessed for radiographic joint changes and loss of joint function to reduce the need for surgery [20].
Knee
With the aging of the population, the number of patients with osteoarthritis of the knee has increased dramatically [41]. In patients with PsA, the knee might be affected early, particularly in patients with asymmetric oligoarthritis or polyarthritis [10, 24]. Knee involvement causes severe pain, restricted movement, instability, and progressive loss of joint function. After hip surgery, knee surgery is the most common orthopedic procedure in patients with PsA [8, 20, 25, 27].
Arthroscopic synovectomy is recommended to minimize surgical trauma in patients with severe knee pain and symptoms of joint inflammation. Arthroscopic synovectomy is feasible in patients with both short- and long-lasting knee joint synovitis, radiographic Larsen grades I–II, and different degrees of cartilage damage [24, 40, 42]. Arthroscopic synovectomy is safe. Combined with pharmacological therapy, it can preserve knee joint motion range and reduce local joint inflammation in patients with PsA [24, 42]. Moreover, good long-term outcomes were observed in patients with PsA with severe cartilage damage in the knee [24]. Long-term outcomes of arthroscopic synovectomy in PsA have not been assessed in large studies. However, arthroscopic synovectomy seems promising because it reduces pain, slows down disease progression, and delays the need for total knee replacement (Figures 2 A, B) [24, 40].
Figure 2
A – A 22-year-old man with pain in the patellofemoral joint. Arthroscopic appearance of a thickened fibrous synovial fold (white star) in the suprapatellar pouch, which conflicted with the patella. B – Arthroscopic view after removal of the synovial fold. After surgery, the patient regained a full pain-free range of movement in the affected knee
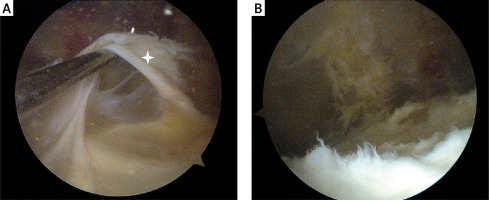
Total knee arthroplasty (TKA) is indicated in patients with end-stage knee destruction, severe pain, and disability [8]. Michet et al. suggested that patients with severe hip arthropathy are more likely to undergo TKA compared with patients without hip involvement [29]. Total knee arthroplasty is done most commonly in patients with asymmetric oligoarthritis, followed by patients with symmetric polyarthritis and peripheral or axial PsA. The mean duration of PsA at the time of TKA is about 8 years [20]. Studies evaluating long-term outcomes of TKA in PsA are few and were done more than two decades ago [43]. It is difficult to compare TKA outcomes between early and recent studies because the enrolled patients differ in local disease severity, type of surgical procedure, and pharmacological treatment [20].
Intra-articular injuries need prompt orthopedic management in patients with PsA. In a meta-analysis, Edd et al. [44] found that intra-articular trauma induces inflammation in joints. This can lead to or accelerate osteoarthritic changes, particularly in patients with predisposing factors, such as inflammatory diseases [36]. Thus, articular damage can progress fast in patients with PsA. Certain conditions, such as rupture of the anterior cruciate ligament or torn meniscus, need faster surgical treatment in patients with PsA compared with healthy people [44] (Figures 3 A, B).
Figure 3
A – An anterior-posterior radiograph obtained in a 69-year-old woman with severe osteoarthrosis of the left knee. There is “fuzzy” bone proliferation in the tibial shaft (white arrow). B – The same view after total knee replacement. Postoperatively, the pain-free range of movement in the knee was 0–120°
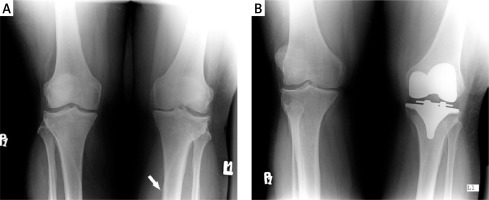
In patients with PsA and knee involvement, surgery is considered when optimal medical therapy has failed or when knee injury occurs. Patients after THA need careful monitoring because they are prone to develop severe knee arthritis, which might require TKA. It is worth adding that some patients with PsA have been treated effectively with a hyaluronic acid based formula [45].
Foot and ankle
Foot abnormalities are observed in 50% to 70% of patients with PsA [46]. Moreover, foot abnormalities, such as dactylitis, tenosynovitis, enthesitis, and joint inflammation, might be the first symptoms of PsA. In the study of Bezza et al. [46], 53% of patients with PsA had isolated hindfoot involvement, and 14% had isolated forefoot involvement. In patients with isolated hindfoot involvement, pain in the inferior or posterior parts of the heel is typical. Clinical manifestations of forefoot involvement include inflammatory metatarsal pain, claw toe deformity, and arthritis mutilans of the toe [46]. Dactylitis is an important symptom of PsA, and it affects feet more commonly than hands [13, 46]. In patients with PsA, dactylitis carries the risk of being a more aggressive disease [14].
There are no clear indications for orthopedic interventions in patients with PsA who have foot abnormalities. Foot orthoses or corrective footwear might help prevent formation of foot deformities. Both in patients with rheumatoid arthritis and those with PsA, splints can stabilize and immobilize the hindfoot and ankle, particularly in patients with arthritis or enthesitis. Functional foot orthoses might correct foot deformities, protect foot joints, and reduce mechanical stress in soft tissues. Enthesitis is treated pharmacologically with NSAIDs, DMARDs, and biologic agents [47]. Moreover, steroids can be injected under ultrasound guidance into the site of the most pronounced inflammation [47].
Surgery can be considered in patients with PsA and foot abnormalities who do not respond well to conservative treatment or have persistent pain. Arthrodesis is the most common surgery used to treat patients with ankle arthropathy. Arthroplasty is indicated in patients with involvement of the forefoot or toe joints (Figures 4 A, B) [8].
Figure 4
A – Subtalar sclerotisation in 30-year old man, B – postoperative lateral radiograph shows Kirchner wires used to preserve articular surface after arthrodesis
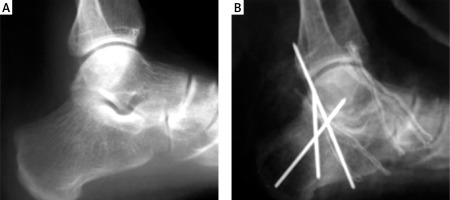
There is little evidence regarding orthopedic operations in patients with PsA who have ankle and foot involvement. In the study of Haque et al., over a 14-year period of observation, only 2 patients with PsA had ankle prostheses, and 40 patients underwent joint-preserving surgery due to foot and hand abnormalities [20]. Hicken et al. [28], during a 15-year period, observed 17 patients with PsA who underwent multiple foot and ankle operations, among which forefoot arthroplasty was the most common. In that study, 89% of the patients were satisfied with surgery outcomes. Moreover, the patients did not have clinical disease progression and did not need additional interventions [28].
Foot or ankle involvement rarely requires surgical intervention in patients with PsA. Among patients with PsA, those with symmetric polyarthritis are more likely to benefit from foot or ankle surgery [27]. In patients with psoriasis, foot involvement might be the first manifestation of PsA, and the development of foot abnormalities requires a change in treatment.
Physiotherapy
Physiotherapy is important in the management of PsA. It may be used in addition to pharmacological treatment, particularly in patients with enthesitis and axial disease [22]. The main goals of physiotherapy are to reduce pain and stiffness, prevent or delay deformity, and restore function in the affected joints. To date, few studies have assessed the place of physiotherapy in PsA [48]. Different exercise regimens aim to restore muscle strength and reduce joint deformity. Different patients with PsA need different physiotherapy approaches [48]. For patients with predominant peripheral PsA, Lubrano et al. proposed a rehabilitation scheme that includes exercises to improve muscle strength and general fitness. The scheme also includes stretching exercises, occupational therapy, and patient education. In patients with predominant axial involvement, postural and breathing exercises are recommended [48].
Pharmacological treatment
Discovery of novel immunologic pathways important for the development of psoriasis or PsA resulted in many new therapies that target those pathways (Table V) [49–52]. These new therapies are considered safe, with common side effects including injection-site reactions and infections (e.g. for adalimumab, etanercept, infliximab), Candida infections (e.g. for ixekizumab, secukinumab) or weight loss and diarrhea (for apremilast) [52]. However, there are still patients with PsA who do not achieve the ACR 20 composite joint outcome [49]. It cannot be excluded that in the future, similar to rheumatoid arthritis, novel therapies will be directed toward molecular and cellular targets [53].
Table V
Summary
Management of PsA should be individualized for each patient. NSAIDs, DMARDs, and biologic agents are the mainstay of treatment for patients with PsA. Surgical treatment can be beneficial in patients with severe and progressive pain, disability, restricted hip movements, hip or knee joint instability, and progressive loss of functional independence. Orthopedic procedures should be considered only when the best medical treatment has failed. Dermatologists, rheumatologists, and orthopedic surgeons should work together to ensure that patients with PsA receive the best possible treatment.


