Colorectal cancer accounts for approximately 10% of all annually diagnosed cancers and cancer-related deaths worldwide. It is the second most common cancer diagnosed in women and the third most common in men [1]. Similarly, gastric cancer (GC) is one of the most common malignancies in the world, with an annual incidence of about 1 million, making it the fifth most common cancer and the third leading cause of cancer-related deaths worldwide [2]. XELOX (intravenous oxaliplatin 130 mg/m2 on day 1, capecitabine 1000 mg/m2 twice daily for 14 days, and repeated every 3 weeks) and SOX (intravenous oxaliplatin 130 mg/m2 on day 1, S-1 40 mg/m2 twice daily for 14 days, and repeated every 3 weeks) are the recommended adjuvant chemotherapy regimens for patients with stage III CRC and stage II-III GC according to the Eighth Edition AJCC Cancer Staging System [3]. Chemotherapy plays an important role in controlling cancer growth and achieving appreciable cancer treatment efficacy [4, 5]. However, chemotherapy, if used for a long term and/or with a high dose, may cause a number of side effects or even toxicity [6]. Chemotherapy-induced thrombocytopaenia (CIT), characterized by an abnormally decreased blood platelet count, is a clinically common chemotherapeutic dose-dependent toxic reaction [7]. CIT mainly occurs in patients treated with taxane-, anthracycline-, and gemcitabine-based regimens recommended by the CIT Chinese Experts Consensus [8, 9]. The prevalence of thrombocytopaenia is 84% and 70.5%, ≥ grade 3 thrombocytopaenia is 9% and 7.5% among CRC and GC patients in East Asia, who received adjuvant XELOX or SOX treatment, respectively [10, 11]. To reduce the incidence of CIT, a secondary prevention strategy has been proposed, in which prophylactic platelet growth factors are administered in the subsequent chemotherapy cycles to prevent severe CIT in patients who are at high risk of bleeding [9]. Currently, recombinant human TPO (rhTPO) has been recommended by the Chinese Food and Drug Administration (SFDA) to treat CIT [9]. Although the application of rhTPO to suppress the occurrence of CIT has been well recognized [12], the most appropriate time for treatment administration remains controversial, particularly because not all chemotherapy drugs cause thrombocytopaenia via the same mechanisms [13]. For example, alkylating agents affect stem cells, cyclophosphamide impacts late megakaryocyte progenitors, bortezomib prevents platelet release from megakaryocytes, and some other treatments promote platelet apoptosis [14]. Thus, the incidence, severity, and duration of thrombocytopaenia varies with chemotherapy regimens. Capecitabine, a commonly used drug to treat CRC patients, is an oral prodrug that is converted to its only active form, fluorouracil, by thymidine phosphorylase (TYMP) [15]. Thus, a polymorphism in the thymidine phosphorylase gene may impact the prognosis or safety of CRC patients who receive capecitabine-based adjuvant chemotherapy by influencing TYMP transcription [16]. Both capecitabine and S-1 (an oral combination of tegafur, gimeracil, and oteracil at a molar ratio of 1 : 0.4 : 1), which are oral fluoropyrimidines, have been proposed as substitutes for continuous infusion of 5-FU because they are more convenient and have lower risks [10, 11]. In contrast, oxaliplatin, a third-generation platinum compound, provides high efficacy in CRC treatment through its ability to form covalent adducts with DNA and recognize and repair Pt-DNA adducts [17]. These drugs are the major components of the XELOX and SOX regimens, which have been shown to cause thrombocytopaenia [11, 18]. However, the optimal timing for the prophylactic administration of rhTPO to prevent XELOX or SOX regimen-induced thrombocytopaenia has not been well explored. In the present study, we conducted a prospective, self-controlled study on rhTPO prevention of CIT in stage III CRC or stage II-III GC patients with grade 2 or more severe CIT according CTCAE v4.0 induced by XELOX or SOX regimen adjuvant chemotherapy.
Patients with grade 2 or more severe CIT induced by XELOX or SOX adjuvant chemotherapy after radical resection of CRC or GC, who were treated at the Ordos Centre Hospital between 1 January 2018 and 31 March 2021 were enrolled in this study. Patients who had all of the following were included in this study: 1) normal platelet count at baseline (≥ 100 × 109/l) ; 2) grade 2 of more severe CIT in the last treatment cycle with recovery to ≥ 85 × 109/l before the next prophylactic cycle; 3) the same regimens and oxaliplatin dose intensification reduction ≤ 10% in the prophylactic cycle; 4) no haematological diseases that influence the blood platelet number; 5) normal liver and kidney function and normal prothrombin time; 6) no severe cardiopulmonary dysfunction; and 7) a Karnofsky physical status (KPS) greater than 80. Patients with one or more of the following were excluded from this study: 1) changed chemotherapy regimen or decreased dose by more than 10% due to severe side effects in the next cycle; 2) poor compliance and dropout; 3) grade 2 or more severe CIT in the last treatment cycle with no recovery to 85 × 109/l before the next prophylactic cycle; 4) haematological system diseases that influence haematopoietic function; 5) recent administration of drugs that influence haematopoiesis; 6) severe respiratory and circulatory diseases; or 7) a KPS of less than 80. After applying the inclusion and exclusion criteria, a total of 30 patients were registered in this study. All patients voluntarily signed informed consent forms. This study complied with the basic standards of the Declaration of Helsinki and was approved by the Institutional Review Board.
The XELOX treatment protocol is described below: oxaliplatin (130 mg/m2, as a one-time dose) was administered on day 1, and capecitabine (1000 mg/m2, given twice a day) was administered on days 1–14, for a total of 21 days, constituting one cycle. The SOX treatment protocol was as follows: oxaliplatin (130 mg/m2, as a one-time dose) was administered on day 1, and S-1 (40 mg/m2, given twice a day) was administered on days 1–14, for a total of 21 days, constituting one cycle. The oral capecitabine or S-1 was interrupted when the platelet count decreased to less than 75 × 109/l and resumed when the platelet count increased after treatment and reached 85 × 109/l. The capecitabine or S-1 doses were fixed, and the oxaliplatin dose decreased by no more than 10%, due to severe side effects during the whole cycle, to better evaluate CIT improvement.
The rhTPO used in this study was a full-length glycosylated molecule (TPIAO, Shenyang 3SBIOINC Co. Ltd., Shenyang, China).
First-time thrombocytopaenia occurrence was defined as the treatment cycle (i.e. the first, second, and third treatment cycle), and the following cycles with the same chemotherapy regimen and dose intensity after the treatment cycle were defined as the prophylactic cycles (i.e. the first, second, and third prophylactic cycle). In the treatment cycle, thrombocytopaenia was treated by subcutaneous injection of rhTPO (15,000 U/day) when the platelet count decreased to less than 75 × 109/l until the platelet count increased to 85 × 109/l. In the prophylactic cycles, a subcutaneous injection of rhTPO (15,000 U/day) was administrated starting on days 4, 3, and 2 before chemotherapy. The experimental groupings were as follows: the treatment cycle was defined as the first, second, and third treatment cycle according the first, second, and third prophylactic cycle, respectively, because not all enrolled patients entered the next prophylactic cycle in this self-controlled study. Routine blood tests were performed at least twice a week after the initiation of the chemotherapy. The number of platelets before and after chemotherapy in the treatment cycle and prophylactic cycles were recorded. If grade 2 or worse thrombocytopaenia after prophylactic medication persisted, more rhTPO was injected or exogenous platelets were infused until the platelet level recovered to the normal level. The following laboratory monitoring indexes were recorded and compared between treatment and prophylactic cycles:
the nadir of blood platelet counts and time of appearance;
the number of days for administration of rhTPO during the treatment cycle and prophylactic cycles;
the number of days of interrupted chemotherapeutic agents in every chemotherapy cycle;
the number of days of TPO treatment for platelet recovery (≥ 100 × 109/l);
the times of exogenous platelet infusion or platelet count less than 10 × 109/l;
adverse event of thrombocytosis after rhTPO injection.
Data are represented as mean ± the standard error of mean (SEM), and a t-test was used to compare data between groups. A p-value less than 0.05 was considered statistically significant. All data analyses were performed using the SPSS17.0 software.
A total of 30 patients with pathology-confirmed stage III colorectal adenocarcinoma or stage II-III gastric carcinoma, who were treated in the Ordos Central Hospital Oncology Department between 1 January 2018 and 31 March 2021 were included in this study. The demographics and basal clinical characteristics of these patients are shown in Table I.
Table I
Patient demographics and basal clinical characteristics
| Variables | N (%) |
|---|---|
| Age: | |
| > 60 | 17 (67.7) |
| < 60 | 13 (32.3) |
| Sex: | |
| Male | 11 (38.7) |
| Female | 19 (61.3) |
| Colorectal Cancer Stage*: | |
| IIIA (T1-T2 N1-N2a M0) | 6 (20.0) |
| IIIB (T1-T4a N1-N2b M0) | 8 (26.7) |
| IIIC (T3-T4b N1-N2b M0) | 7 (23.3) |
| Gastric Cancer Stage*: | |
| IIB (T1-T4a N0-N3a M0) | 3 (10.0) |
| IIIB (T1-T4b N1-N3b M0) | 6 (20.0) |
| Histology: | |
| Colon adenocarcinoma cancer | 12 (40.0) |
| Rectal adenocarcinoma cancer | 8 (26.7) |
| Gastric adenocarcinoma cancer | 10 (33.3) |
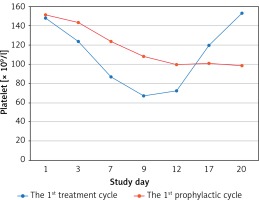
All enrolled patients had normal platelet count at baseline (≥ 100 × 109/l), and all finished their planned chemotherapy dose intensification without reduction during the chemotherapy course. In the first treatment cycle, 2 (6.7%) patients suffered grade 4 thrombocytopaenia, 9 (30.0%) patients had grade 3 thrombocytopaenia, and 19 (63.3%) patients had grade 2 thrombocytopaenia. In the first prophylactic cycle, 1 (3.3%) patient suffered grade 4 thrombocytopaenia, 3 (10.0%) patients had grade 3 thrombocytopaenia, and 11 (36.7%) patients had grade 2 thrombocytopaenia. The primary objective nadir of platelet count was significantly higher in the first prophylactic cycle compared to the first treatment cycle (75.60 ±26.96 × 109/l vs. 53.37 ±16.86 × 109/l, p < 0.001). In addition, the first prophylactic cycle had a significantly shorter interrupted time for oral capecitabine or S-1, shorter duration of rhTPO administration, and shorter platelet recovery time compared to the first treatment cycle, and all reached statistical significance (Table II). One patient received platelet transfusion during the first treatment, and no platelet transfusion was performed during subsequent cycles. The curves of platelet count changes in the first treatment and first prophylactic cycles are shown in Figure 1.
Table II
Comparison of clinical and laboratory indexes between the first treatment and the first prophylactic cycle
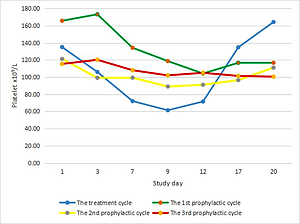
Figure 1
Platelet count curves of the first treatment (blue line) and the first prophylactic (red line) cycle
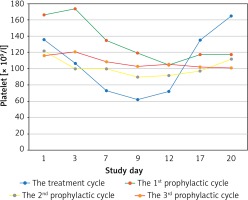
Most patients’adjuvant chemotherapy plans included 6 cycles, because of the median cycles for grade 2 CIT occurred was the fourth cycle, there were 7 and 4 patients who received continuous rhTPO prophylactic treatment during the second and the third prophylactic cycles according to the treatment plan and patients’ preference, respectively. There were only 3 patients who developed ≥ grade 3 thrombocytopaenia during the 2nd and the 3rd prophylactic cycle, while 8 patients developed ≥ grade 3 thrombocytopaenia during the second and third treatment cycles. While the nadir platelet counts were significantly higher than in the second and third prophylactic cycles (69.56 ± 18.26 × 109/l vs. 52.88 ±16.07 × 109/l, p = 0.013; 82.33 ±20.90 × 109/l vs. 52.83 ±14.58 × 109/l, p = 0.037). There was a shorter duration of rescue treatment in the second prophylactic cycle compared to the second treatment cycle (1.63 ±2.03 vs. 3.06 ±1.57, p = 0.035). It tended to have a shorter duration of interruption of chemotherapy and platelet recovery in the second prophylactic cycle compared to the second treatment cycle because there were no statistically significant differences detected (Table III). The third prophylactic cycle had a significantly shorter duration of rescue treatment and platelet recovery compared to the third treatment cycle; however, no significant difference was observed in the number of days of interruption of chemotherapy between these 2 groups (Table IV). No patients received exogenous platelets in the continuously rhTPO prophylactic cycle. The change curves of platelet count are shown in Figures 2–4.
Table III
Comparison of clinical and laboratory indexes between the second treatment and the second prophylactic cycles
Table IV
Comparison of clinical and laboratory indexes between the third treatment and the third prophylactic cycle
Figure 2
Platelet count curves of the second treatment (blue line) and the second prophylactic (red line) cycle
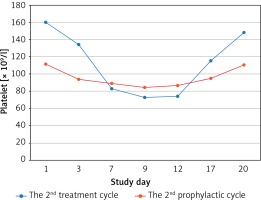
Figure 3
Platelet count curves of the third treatment (blue line) and the third prophylactic (red line) cycle
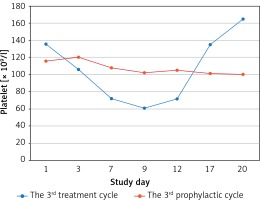
Figure 4
Platelet count curves of the treatment (blue line) and all prophylactic cycles (first prophylactic cycle – green line; second prophylactic cycle – yellow line; third prophylactic cycle – purple line)
No thrombocytosis complications were observed in patients during the treatment and prophylactic cycles.
In the present study, we investigated the efficacy of rhTPO prophylactic treatment for the prevention of CIT induced by XELOX or SOX regimen adjuvant chemotherapy in stage III CRC or stage II-III stage GC patients. We found that the rhTPO prophylactic treatment effectively reduced the occurrence and severity of CIT in the CRC or GC patients included in this study.
XELOX or SOX are standard adjuvant chemotherapy regimens for CRC or GC patients, especially for patients with stage III CRC or stage II-III GC who are in a higher risk group (i.e. T1-4, N0-2c, or both) [11, 19, 20]. Current studies have shown that capecitabine and S-1 were associated with a high incidence of thrombocytopaenia in a Chinese population [10, 11]. The median nadir of platelet count occurs on day 9 during the period of XELOX or SOX adjuvant chemotherapy, during which time oral fluorouracil agents should be paused. However, adjuvant high-dose chemotherapy has been shown to be correlated with longer overall survival (OS) or improved free recurrence survival (RFS) [21–23]. rhTPO is a specific platelet recovery agent that directly regulates the differentiation of bone marrow haemopoietic stem cells [24]. Clinical research has suggested that rhTPO usually takes 5 days to be effective and takes 10–14 days to reach the peak serum concentrations [24]. Considering that capecitabine 1000 mg/m2 or S-1 40 mg/m2 given twice a day were administered on days 1–14 during XELOX or SOX adjuvant chemotherapy, it is reasonable, for secondary prevention, to administer rhTPO on days 4, 3, and 2 before chemotherapy.
Previously, prophylactic use of rhTPO has been shown to alleviate the severity and reduce the duration of thrombocytopaenia [25, 26]. Shun et al. reported that prophylactically administered rhTPO on days 2, 4, 6, and 9 of secondary prevention of CIT in subsequent prophylactic cycles reduced the occurrence of CIT induced by a GP regimen in lung cancer patients [26]. Another study suggested that it is important to administer rhTPO between days 4 and 5 after chemotherapy, combining isocyclophosphamide with anthracycline [27]. In the present study, we prophylactically administrated rhTPO on days 4, 3, and 2 before chemotherapy and obtained appreciable clinical outcomes similar to what has been previously reported [26, 27]. We found that the mean nadir of platelet count in all prophylactic cycles was significantly higher than that in the treatment cycle, which correlated with a reduced number of administration days of rhTPO and days of platelet recovery, as well as alleviated CIT severity. The interrupted time in chemotherapy cycles was also significantly decreased in the first prophylactic cycle compared to the first treatment cycle. Thus, we speculate that prophylactic administration of rhTPO on days 4, 3, and 2 before chemotherapy is appropriate to attenuate thrombocytopaenia induced by XELOX regimen in CRC patients or SOX regimen in GC patients. Most patients’ adjuvant chemotherapy plans were 6–7 cycles in total, because the median cycle for the development of grade 2 or more thrombocytopaenia was the fourth cycle. Our findings provide evidence that the appropriate timing of rhTPO administration can ensure the dose intensity and duration of XELOX or SOX regimen adjuvant chemotherapy.
Previous studies on the secondary prevention of thrombocytopenia by prophylactic administration of rhTPO mainly compared the treatment cycle and the first prophylactic cycle [26–28]. In the present study, we compared the changes in the laboratory indexes in patients who continuously received the prophylactic rhTPO treatment in all 3 prophylactic cycles. We observed that group C had an increased mean nadir of platelet count, and reduced number of the administration days of rhTPO and days of platelet recovery ≥ 100 × 109/l, as well as alleviated severity of CIT. We noted that there were no statistical differences between some subgroups, particularly between the control group and group B. We speculate that this was probably due to the decreased patient numbers in the continuous prophylactic cycles and the accumulation of chemotherapeutic toxicity. To further examine the possible mechanisms, we need to select an optimal dose of rhTOP that 1) can increase the number of bone marrow megakaryocytes and 2) increase the reservoir of platelet precursors before chemotherapy. Indeed, the number of megakaryocytes was still significantly higher than baseline, and the cells were largely mature and polyploid in the rhTOP prophylactic treatment group [27], indicating that rhTPO protected megakaryocytes from chemotherapy-induced apoptosis. This notion is supported by prior observations that rhTPO is a survival factor and prevents programmed cell death in megakaryocytes and progenitors in vitro [28, 29].
Some limitations of this study need to be discussed: 1) This was a single-centrr study with a small sample size; hence, our findings should be further corroborated in a multi-centre study with a larger cohort in the future; 2) In the present study, we did not examine the incidence of other side effects except thrombus; and 3) Our data warrant further comparison of the prophylactic effects of rhTPO administration between different secondary prophylactic regimens.
In conclusion, we report here that the prophylactic administration of rhTPO on days 4, 3, and 2 before chemotherapy can significantly attenuate CIT occurrence and severity induced by a XELOX regimen in CRC patients or a SOX regimen in GC.