Introduction
Chronic liver disease represents a significant global public health challenge, exerting a profound impact on individual well-being and social economies [1]. The World Health Organization (WHO) reports that hundreds of millions of individuals worldwide grapple with diverse chronic liver diseases, with liver fibrosis emerging as a common terminal pathway for many of these conditions. The progression of liver fibrosis may culminate in cirrhosis or even hepatocellular carcinoma (HCC), causing a grave threat to patient safety [2, 3]. Therefore, timely diagnosis and monitoring of liver fibrosis hold paramount importance for effective disease management and prognosis. Major contributors to chronic liver diseases include viral hepatitis (such as hepatitis B and C), alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD), and autoimmune liver disease. These conditions progressively inflict damage on the liver, inducing inflammation and fibrosis. As the diseases advance, liver tissue undergoes replacement by scar tissue, losing its normal functionality. Studies underscore that chronic liver disease and its complications rank among the leading causes of global mortality. The burden it places on public health systems, particularly in resource-limited regions, is notably prominent.
Traditionally, liver biopsy serves as the “gold standard” for diagnosing liver fibrosis, offering a direct observation of liver tissue changes. However, this method is invasive, harbors the risk of complications, and is susceptible to sample errors [4]. Consequently, there is a pressing need to explore non-invasive alternatives, prompting ongoing research in this area. In addition, certain biomarkers, including serological indicators (such as liver function indicators, platelet count, etc.) and fibrosis-specific markers (such as hyaluronic acid, laminin, etc.), are employed to assess the degree of liver fibrosis. While changes in these biomarkers can reflect the liver’s fibrosis state, they are often influenced by various factors, limiting their accuracy and specificity, and making it challenging to accurately reflect the actual degree of fibrosis. Despite the partial effectiveness of traditional diagnostic methods and biomarkers in evaluating liver fibrosis, their inherent limitations necessitate the exploration of new, non-invasive approaches. Elastography, as a novel diagnostic method, is gradually garnering attention for its role in assessing liver fibrosis in chronic liver disease [5]. This article aims to comprehensively review the recent advancements in elastography for fibrosis assessment in chronic liver disease, exploring its potential and addressing the challenges it presents as a substitute for quantitative biomarkers in gauging the burden of liver fibrosis.
Epidemiology and pathophysiology of chronic liver disease
Chronic liver disease stands as a formidable health challenge, involving various disease states such as chronic viral hepatitis, alcoholic liver disease, NAFLD, autoimmune liver disease, and hereditary conditions like Wilson’s disease and hemochromatosis. These diseases inflict prolonged damage on the liver’s structure and function, potentially progressing to cirrhosis, liver failure, and even liver cancer. It remains a major worldwide health concern, with the WHO reporting approximately 324 million people affected by chronic hepatitis B and 158 million by chronic hepatitis C. The incidence of alcoholic liver disease and NAFLD is on the rise, especially in Western countries, with NAFLD becoming the most prevalent chronic liver disease, affecting around 25% of the global population. Furthermore, regional disparities exist in the distribution of chronic liver diseases; for example, chronic hepatitis B is more prevalent in Asia and sub-Saharan Africa, while hepatitis C is predominant in Eastern Europe and the Mediterranean. Alcoholic liver disease is more commonly observed in Europe and North America.
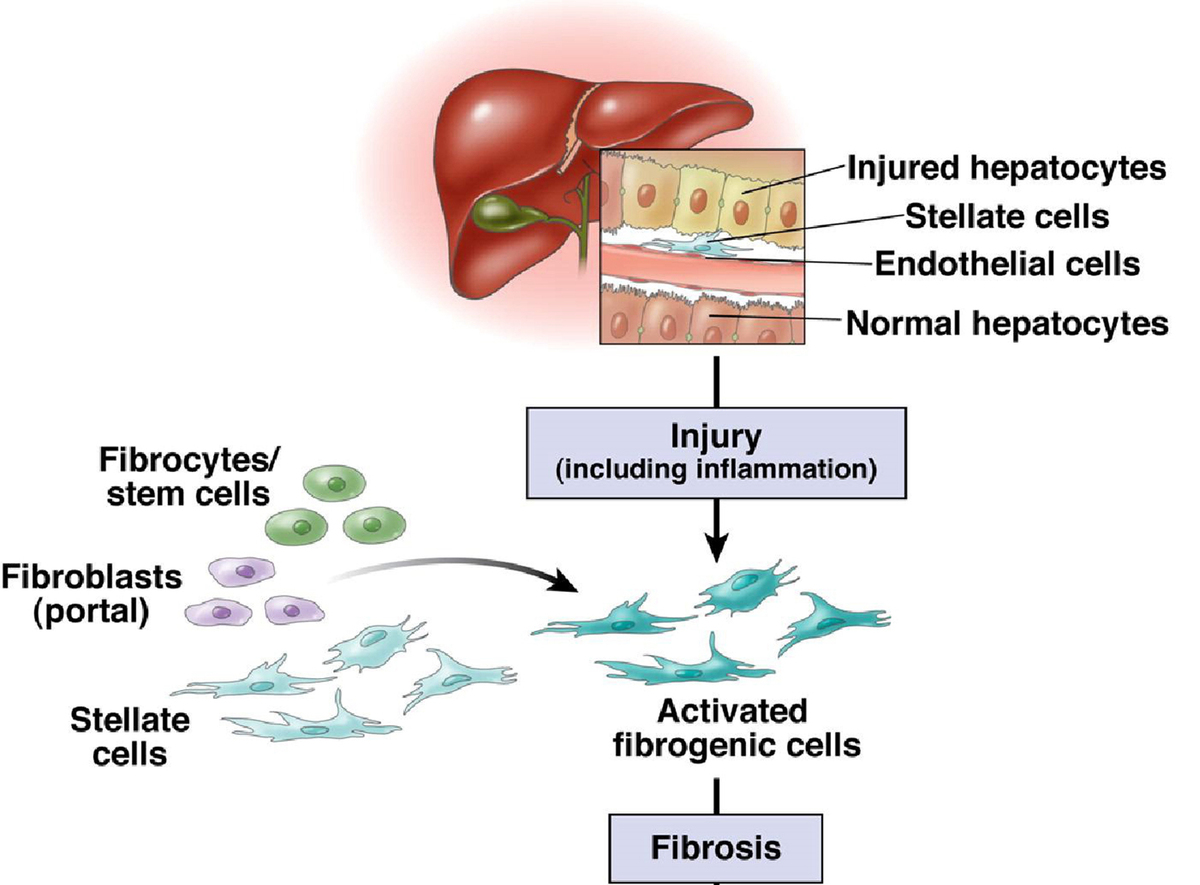
The pathophysiological cascade of chronic liver disease typically commences with prolonged liver inflammation triggered by factors such as virus infections, abnormal metabolism, excessive alcohol consumption, and exposure to drugs or toxins [6]. This inflammatory response can result in damage to liver cells, activating hepatic stellate cells. The activation of these cells prompts the production of significant amounts of collagen and other extracellular matrix components, leading to the development of liver fibrosis. Over time, persistent liver fibrosis may progress to cirrhosis, making the stage of irreversible structural changes in the liver. Cirrhosis is characterized by the excessive deposition of connective tissue, causing restructuring and angiogenesis within the liver. These alterations significantly impact liver hemodynamics and function, potentially resulting in portal hypertension and hepatic insufficiency. Moreover, individuals with liver cirrhosis face an elevated risk of developing hepatocellular carcinoma (HCC), one of the most severe complications of chronic liver disease. HCC ranks as the sixth most common cancer and the third leading cause of cancer-related deaths globally [7, 8]. The course and prognosis of chronic liver disease hinge on diverse factors, including the underlying cause of the disease, patients’ baseline health status, the emergence of complications, and the timeliness and effectiveness of treatment. Identifying and treating the early stages of chronic liver disease is pivotal in preventing the progression of liver fibrosis and enhancing patient prognosis.
Liver fibrosis and its evaluation methods
Definition, harm, and developmental stage of liver fibrosis
Hepatic fibrosis serves as a pivotal stage in the progression of chronic liver disease, playing a crucial role in the understanding and management of various liver disorders. This pathological reaction is a response to long-term liver injury, usually induced by chronic inflammation [9, 10]. Central to this process is the activation of hepatic stellate cells, which typically store vitamin A and support the basic functions of the liver. Under the influence of chronic injury, these cells undergo transformation into myofibroblast-like cells, generating an abundance of collagen and other extracellular matrix components. The deposition of these components in the liver results in the replacement of normal liver tissue structure with fibrous tissue. Hepatic fibrosis serves as a critical indicator of the progression of chronic liver disease, signaling a shift from a mild inflammatory state to a more severe condition that may precipitate serious complications. As fibrous tissue accumulates, the normal structure and vascular arrangement of the liver are disrupted, significantly compromising its function. The liver, being the primary metabolic organ responsible for detoxification, drug metabolism, and the synthesis of essential proteins like coagulation factors, experiences a considerable reduction in these functions as fibrosis progresses. Persistent liver fibrosis can advance to cirrhosis, an irreversible state characterized by severe damage to the liver structure and a profound reduction in liver function. Hepatic fibrosis heightens the risk of severe complications, such as portal hypertension, ascites, esophageal variceal bleeding, and hepatic encephalopathy. These complications substantially increase the mortality risk for affected individuals. Long-term liver fibrosis also emerges as an important risk factor for the development of HCC, a highly malignant liver tumor closely related to liver fibrosis and cirrhosis. The primary infection factor contributing to liver fibrosis is various forms of viral hepatitis. Upon infection with these viruses, individuals may develop a range of infectious diseases, characterized by liver inflammation and necrosis. Viral hepatitis exhibits strong infectivity, complex transmission routes, and widespread prevalence, leading to a high incidence rate. Additionally, drug-induced hepatitis, stemming from the liver’s role as the primary site of most drug metabolism, can cause direct or indirect toxic damage to liver cells. This occurs due to the metabolism of certain drugs within the liver. Common clinical reactions include heterogeneous reactions, with drugs such as Polygonum multiflorum and nonsteroidal anti-inflammatory and analgesic drugs to cause liver damage.
Accurate identification and assessment of the degree of hepatic fibrosis are crucial components of chronic liver disease management. Traditionally, liver biopsy has been employed to evaluate fibrosis severity, but this method is invasive and causes potential complications. In recent years, non-invasive imaging techniques such as elastography have emerged as safer and more convenient alternatives [11, 12]. The overarching goal of treating chronic liver disease and liver fibrosis is to decelerate or halt disease progression and, where possible, reverse existing fibrosis. This may involve antiviral therapy, moderating alcohol intake, weight and blood sugar control, and the use of specific drugs to impede the fibrosis process. For patients already diagnosed with cirrhosis, the emphasis is on monitoring and preventing complications. The importance of liver fibrosis in chronic liver disease cannot be overstated – it is not only a crucial stage in disease progression but also the primary driver behind declining liver function and the onset of severe complications. Hence, timely identification, accurate evaluation, and effective treatment of liver fibrosis constitute the core elements of chronic liver disease management. With a deepened understanding of the mechanisms underlying liver fibrosis and the application of innovative technologies, the prospect of more effective methods to arrest and reverse the procession of liver fibrosis is promising.
Evaluation methods for liver fibrosis
Non-invasive evaluation methods for liver fibrosis: advantages and limitations of quantitative biomarkers in diagnosis
In the evaluation of liver fibrosis, various biomarkers, including serological indicators (such as liver function indicators, platelet count, etc.) and fibrosis-specific markers (such as hyaluronic acid, laminin, etc.) are utilized. While changes in these biomarkers can offer insights into the liver’s fibrosis state, their accuracy and specificity are often compromised by numerous influencing factors, making it challenging to accurately gauge the actual degree of fibrosis. Quantitative biomarkers play an important role in the diagnosis and monitoring of liver fibrosis [13]. These markers, including serum hyaluronic acid, laminin, type IV collagen, among others, furnish direct chemical indicators of the liver’s pathological state through blood tests. Their primary advantage lies in their ability to reflect the inflammatory activity and fibrosis procession of the liver, especially in the early stages of liver disease. In addition, biomarker testing is typically straightforward, rapid, nearly non-invasive, and causes an extremely low risk to patients. However, quantitative biomarkers are not without their limitations. First of all, the levels of these markers can be influenced by a multitude of physiological and pathological factors, including age, sex, weight, drug use, and other patient complications. Therefore, when interpreting biomarker levels, due consideration should be given to these influencing factors. Secondly, a single biomarker often lacks the diagnostic accuracy required, necessitating the combination of multiple biomarkers or the utilization of composite algorithms to improve diagnostic sensitivity and specificity.
Invasive evaluation methods for liver fibrosis
Liver tissue biopsy remains the gold standard for determining the degree of liver fibrosis and inflammation, playing an important role in clinical practice. However, it is an invasive procedure associated with inherent risks, making it challenging for patients to accept and limiting its widespread clinical utility. The limitation hampers timely interventions in patient treatment scenarios. Additionally, the subjective judgments of pathologists may arise if the size of liver tissue biopsy only represents a fraction of the total liver volume. These factors collectively impact the accuracy of pathological examinations and consequently influence the overall evaluation of liver fibrosis disease.
The principle, classification, advantages, disadvantages, and application scope of elastography
Basic principle of elastic imaging technology
Elastography, a non-invasive medical imaging technology, is employed to measure and visualize the mechanical properties and elasticity of tissues. At its core, this technique relies on the fundamental principle that various tissue types (such as normal tissue, inflammatory tissue, or tumors) exhibit distinct elastic properties. By measuring the tissue’s response to an external force, typically sound waves, elastography provides valuable insights into tissue hardness, facilitating the diagnosis of various diseases. The basic principle involves inducing tiny displacements of tissues through external forces (such as sound waves) and detecting these displacements using ultrasound equipment [14]. Tissue elasticity is then inferred by quantifying these displacements, with harder tissues, like fibrotic liver tissues or certain tumors, showing less displacement, while softer tissues exhibit greater displacement.
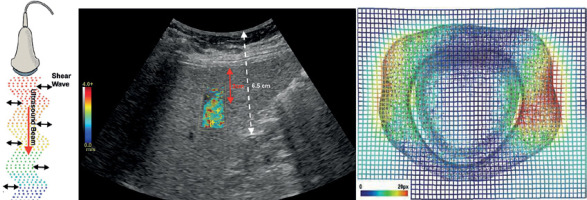
The essence of elastic imaging technology lies in utilizing external forces, usually sound waves, to induce tiny tissue displacements and using ultrasound equipment to detect these displacements. This method allows physicians to evaluate and visualize the elastic properties of tissues, providing important information for the diagnosis and monitoring of diverse medical conditions. The indirect evaluation of tissue hardness is a pivotal aspect of this technique, with broad clinical applications. In the elastography process, ultrasonic equipment generates sound waves that propagate through the tissue, causing minute displacements. The magnitude and speed of these displacements serve as indicators of the tissue’s elastic characteristics. Detection of these displacements is typically executed by the same ultrasonic equipment, capturing tissue responses to sound waves and generating elastic images (Figure 1).
Classification, advantages and disadvantages of elastography
The classification of ultrasound elastography includes pressure elastic imaging, shear wave elastography, and pulse radiation force elastography. Pressure elastic imaging is accomplished by utilizing elastic imaging software on the instrument, positioning the probe at the patient’s lesion site, and continuously applying pressure. This method utilizes the elastic parameters formed by automatic pressure at the speed of sound. Shear wave elastography, which encompasses encircling meaningful areas with shear waves, finds predominant usage in thyroid and breast organs, boasting an exceptionally high specificity exceeding 81%. Pulse radiation force elastography, reliant on the characteristics of sound beam speed, radiation range, and sampling mode, is currently not widely adopted.
Elastography offers notable advantages in disease diagnosis. Tissues elasticity, or hardness, closely correlates with the biological characteristics of lesions, offering a significant diagnostic value. Ultrasound elastography, as a cutting-edge technology for imaging tissue elasticity or hardness, has become a focal point in clinical research. This innovative imaging nodality expands the connotation and scope of ultrasound diagnosis theory, mitigating the limitations of conventional ultrasound. It vividly displays, locates, and differentiates lesion nature, thereby enhancing modern ultrasound technology. Termed as the E-mode ultrasound mode, it complements existing ultrasound modalities (A-type, B-type, D-type, and M-type). However, elastography is not without its drawbacks. It may be susceptible to cavities, particularly evident in liquefaction necrosis area in two-dimensional shear wave elastography. This occurrence arises from the inability of shear waves to propagate in liquids, adversely affecting image quality and measurement accuracy. Additionally, calcification lesions can inflate measurement results, surpassing the true hardness of lymph nodes. Hence, the drawback of two-dimensional shear wave elastography lies in the presence of voids.
Comparison of different types of elastography techniques
The evolution of elastic imaging technology has ushered in innovations in medical imaging, especially in evaluating the mechanical properties of tissues. Two prominent techniques in this domain are transient elastography (TE) and shear wave elastography (SWE). TE, an early elastic imaging technique, is primarily employed to evaluate liver hardness. It generates elastic waves that traverse the liver by applying a short mechanical pulse to the body surface. The measurement of wave velocity is then used to deduce tissue hardness – faster wave velocity indicates higher tissue hardness, potentially signifying increased fibrosis or hardening. TE is lauded for its simplicity and rapid operability, rendering it highly practical in clinical settings. It has gained popularity as a non-invasive method for the evaluation of liver diseases, especially liver fibrosis [15]. However, TE’s drawbacks include low accuracy in evaluating deep tissues and large tumors, as well as its susceptibility to operator technical proficiency. Additionally, its application in obese patients or those with pleural effusion is limited.
SWE stands out as a relatively recent elastic imaging technology that employs ultrasonic beams to generate and detect shear waves. In contrast to TE, SWE offers a broader area of measurement and generates more detailed elastic images. Regarded as superior to TE in terms of accuracy and repeatability, especially for evaluating deep tissues and large tumors, SWE provides a key advantage by offering quantitative elastic information. This makes it a potent tool for evaluating disease progression and treatment response. SWE’s diminished reliance on operator proficiency contributes to more consistent and reliable results. However, its limitations include a higher cost and dependence on specific types of ultrasonic equipment, along with potential comparability issues between different manufacturers.
The choice between TE and SWE typically hinges on specific clinical scenarios, available equipment, budget constraints, and required accuracy. For swift preliminary assessments, particularly in resource-limited settings, TE may be a more fitting choice. Conversely, SWE proves ideal for situations demanding higher precision and quantitative analysis, such as evaluating or monitoring deep tumors. The ongoing technological advancements in these elastic imaging technologies extend their application beyond liver disease evaluation to encompass diagnoses in the breast, thyroid, prostate, and musculoskeletal system. The progress in these technologies opens up new possibilities for diagnosis, treatment planning, and disease monitoring.
Role and advantages of elastic imaging in quantitative evaluation of liver fibrosis
Elastic imaging technology boasts key advantages, including its non-invasiveness and straightforward operation, providing rapid insights into liver hardness within minutes. Particularly suited for situations requiring frequent monitoring of liver fibrosis progression, such as during antiviral therapy or other liver disease treatments, these techniques enable a comprehensive evaluation of the entire liver, sidestepping the sampling error issues associated with traditional liver biopsy [16, 17]. Another significant advantage is the ability of elastography to furnish information about the distribution of liver hardness across the entire organ, mitigating sampling errors prevalent in liver biopsy. This proves especially valuable in evaluating the distribution and uniformity of liver fibrosis. Additionally, with technological advancements, the accuracy and reliability of elastography continue to improve, solidifying its role as a pivotal tool in the quantitative evaluation of liver fibrosis.
Recent studies have demonstrated the high accuracy of elastography in diagnosing moderate to severe liver fibrosis. For instance, certain studies have found that TE and SWE exhibit strong concordance with liver biopsy outcomes in the detection of significant fibrosis and cirrhosis. In recent years, research has focused on improving the accuracy and usability of elastography techniques. Advanced methods, including SWE and MRE, have been developed to provide more detailed images of liver hardness distribution than traditional TE, thus improving the accuracy of evaluation. Magnetic resonance elastography (MRE), in particular, has proven highly effective in evaluating liver fibrosis, including early-stage changes. The ongoing development of automation and standardization is under way to reduce operator variability and improve result consistency across different devices. Simultaneously, advancements in algorithms and software contribute to the increased accuracy of elastography in providing quantitative data.
Beyond diagnostics, elastography exhibits considerable potential in monitoring the response to liver disease treatment. Regular monitoring of the degree of liver fibrosis is crucial for evaluating the therapeutic effect and adjusting treatment plans, especially for patients with chronic liver disease undergoing antiviral or anti-fibrosis treatment [18, 19]. Elastic imaging technology, with its non-invasive nature, provides an ideal tool for tracking changes in liver hardness. For example, antiviral therapy has been proven to slow down or even reverse the progression of liver fibrosis. Employing TE or SWE to monitor liver hardness changes allows doctors to evaluate treatment effectiveness promptly and make timely adjustments to the treatment plan. This application extends beyond chronic viral liver disease to include alcoholic liver disease, nonalcoholic fatty liver disease, and various forms of chronic liver disease. As treatment methods evolve, the role of elastography in monitoring the therapeutic effects of liver diseases is poised to further enhance.
Elastic imaging technology surpasses quantitative biomarkers in several crucial aspects. Firstly, compared to traditional quantitative biomarkers relying on blood samples, elastography is non-invasive, evaluating liver fibrosis by measuring the elastic characteristics of liver tissue. This eliminates the discomfort and potential infection risks associated with blood collection [20–22]. Secondly, elastic imaging technology provides real-time and dynamic tissue information, enabling direct observation of liver hardness during the examination, enhancing the accuracy of disease progression and treatment effect assessment. This immediate feedback capability is unparalleled compared to quantitative biomarkers, which often requires lengthy laboratory processing and analysis. Additionally, elastography excels in simplicity and cost-effectiveness, usually implemented as an additional function in ultrasonic examinations without requiring complicated equipment or incurring high reagent costs. This ease of integration facilitates widespread adoption in medical institutions of all levels, especially in resource-limited areas [23, 24]. Lastly, from the patient’s perspective, elastography, being a painless, radiation-free, and non-special preparation examination method, significantly improves patient acceptance and compliance. This is especially important for patients with chronic liver disease who require long-term monitoring, ensuring timely examinations to identify changes in their condition and adjust the treatment plans accordingly (Figure 2).
Research progress of liver elastography in the diagnosis of CLD
FibroScan (FS) serves not only as a diagnostic tool for liver fibrosis and cirrhosis but also holds promise in predicting other liver-related diseases [25]. Despite ongoing research, its clinical applications remain limited [25]. However, FS exhibits distinct advantages in diagnosing portal hypertension, esophageal varices, and ascites. Notably, a strong correlation exists between hepatic vein pressure gradient (HVPG) and liver stiffness measurement (LSM), offering valuable insights into surgical planning and alternative therapies for liver cancer patients. When HVPG is less than 10 mm Hg (r = 0.81, p = 0.0003) or 12 mm Hg (r = 0, p = 0.0001), LSM demonstrates robust correlation, aiding in the prediction of portal hypertension with high sensitivity (97%) and AUROC value (0.99) when LSM is 13.6 kPa. However, this correlation diminishes when HVPG exceeds 12 mm Hg, possibly due to the shift in portal hypertension’s etiology from extracellular matrix deposition to intrahepatic hemodynamic changes [25–29].
Comparison of clinical value between elastography and other liver fibrosis assessment methods
Ultrasound elastography technology offers several advantages, including non-invasive, real-time imaging, and ease of operation, revolutionizing tissue hardness parameter assessment in ultrasound diagnosis. SWE enables simultaneous grayscale and elastic imaging, accurately locates regions of interest (ROI), and quantitatively measures elastic modulus values within the ROI. Although TE was an early contender in the evaluation of liver fibrosis, its efficacy is limited by factors such as ascites and obesity, lacking real-time two-dimensional grayscale guidance. Conversely, STE, guided by real-time grayscale images, provides satisfactory elastic images, facilitating easier detection and yielding higher success rates. Research suggests that SWE exhibits superior performance compared to four serum non-invasive diagnostic models – APRI, FIB-4, Forns index, and King’s score – highlighting its higher applicability in liver fibrosis diagnosis over serum indicators. In comparison with liver biopsy, ultrasound elastography boasts non-invasiveness, high patient acceptance, and stability, thereby holding a significant clinical value and warranting broader adoption.
There is a study comparing the clinical diagnostic value of instantaneous elastography (FibroScan) and serological scoring model (APRKFIB-4) for the degree of liver fibrosis. It was found that FIB-4 cannot diagnose significant liver fibrosis, while FibroScan and APRI have better diagnostic capabilities for significant liver fibrosis and early cirrhosis. Therefore, the FIB-4 index has a poor efficacy in evaluating significant liver fibrosis in patients with chronic hepatitis B. FibroScan and APRI scores have good diagnostic and early liver cirrhosis recognition performance, but they still cannot completely replace liver tissue viability. MRE combined with FIB-4 (MEFIB) index can be used to screen non-alcoholic fatty liver related liver fibrosis patients who require drug treatment, with the advantages of non-invasive and high positive predictive value.
Summary and outlook
Elastography, especially TE and SWE, has proven to be a valuable tool for evaluating the degree of liver fibrosis. These techniques indirectly assess fibrosis by measuring liver tissue hardness, providing a non-invasive, rapid, and repeatable evaluation method for healthcare professionals. Recent advancements in research indicate that elastography has achieved high accuracy in diagnosing moderate to severe liver fibrosis [30]. Furthermore, the ongoing technological development, exemplified by the emergence of MRE, has further enhanced the accuracy and reliability of elastography in the evaluation of liver fibrosis [31, 32]. The development of automation and standardization is narrowing the variability between operators, improving result consistency across different devices, and boosting diagnostic reliability [33, 34]. Simultaneously, the evolution of new algorithms and software is improving the accuracy and granularity of elastography, especially in diagnosing early liver fibrosis. These technological strides provide crucial support for managing patients with chronic liver disease, enabling more precise evaluation of disease severity and progression [35, 36]. This, in turn, facilitates the formulation of personalized and effective treatment programs for patients.
The trajectory of elastography in liver fibrosis assessment displays immense potential [37, 38]. Anticipated technological progress is poised to enhance the precision and reduce the cost of operating elastic imaging equipment, broadening its application in various medical settings [39, 40]. Especially in resource-limited areas, this non-invasive and cost-effective diagnostic tool is expected to greatly improve the diagnosis and treatment of liver diseases. The integration of artificial intelligence and machine learning holds promise for further improving the quality and speed of image analysis, enabling automatic liver fibrosis assessments, and enhancing the efficiency and consistency of diagnoses. Furthermore, the synergistic use of elastography with other imaging techniques (such as PET and CT) and biomarkers promises to provide more comprehensive information for the holistic evaluation of liver diseases. This multi-modal imaging approach may assume a pivotal role in future clinical practice, especially in the evaluation of complex or advanced liver diseases. Elasticity imaging technology demonstrates significant potential in the assessment of fibrosis burden of chronic liver disease, and its future development is expected to bolster its role in liver disease diagnosis, treatment monitoring, and overall disease management. With ongoing technological advancements and the emergence of new methods, elastography is poised to play an increasingly substantial role in the diagnosis and treatment of patients with chronic liver disease, providing healthcare professionals and patients with more choices and improved treatment outcomes.