Introduction
Chronic heart failure (HF) represents a major global public health problem. In the United States, approximately 6.2 million adult Americans have chronic HF, with estimations that show an increase to more than 8 million people in 2030 [1]. Similar findings were also observed in European populations [2, 3]. Despite important advances in diagnosis and management over the past two decades [4], chronic HF patients still have a poor prognosis [1, 5–8] with a mortality around 50% at 5 years [9].
Lung congestion is one of the most common signs of HF, being also the leading cause for hospitalisation in adult patients over 65 years from the United States [10]. Traditionally, lung congestion has been evaluated by clinical examination or thoracic radiography, but in recent years lung ultrasonography (LUS) has emerged as a superior diagnostic test in HF patients [11]. Furthermore, LUS, through the assessment of the B-lines, is also a promising tool for monitoring changes in lung congestion [12] and for prognostic stadialisation [12, 13] in these patients. The B-lines are discrete laser-like vertical hyperechoic reverberation artefacts that arise from the pleural line, extend to the bottom of the screen without fading, and move in tandem with lung sliding [14], originating most often from volumetric variations in the relationship between the aerated and tissue/fluid-filled parts of the lung [15].
Bioimpedance spectroscopy (BIS) represents an objective tool that is used to assess and monitor fluid status. It is validated against “gold-standard” methods [16, 17], and its use in dialysis patients has been associated with improved survival [18], better blood pressure control [19], and reduced arterial stiffness [18].
The aim of the study was to evaluate the relationship between lung congestion (as assessed by LUS), BIS-derived and echocardiographic parameters, and to determine the effect of these associations on all-cause mortality in HF patients.
Material and methods
Patients and study design
This was a prospective observational study of outpatient adults referred for clinically indicated transthoracic echocardiograms at an academic hospital between 2016 and 2018. Eligible patients with a left ventricular ejection fraction (LVEF) below 45% were identified via the daily echocardiography assessments. From a total of 264 eligible patients, we excluded 127 patients because of limb amputation (n = 3), metallic joint prostheses (n = 10), cardiac pacemakers or stents (n = 78), decompensated cirrhosis (n = 5), prior diagnosis of pulmonary fibrosis (n = 4), pneumectomy (n = 1), massive pleural effusion (n = 6), end-stage renal disease (n = 3), active systemic infections (n = 4), and terminal illnesses (n = 13). There were an additional 15 patients who refused to participate and were not included in the study. Details of the final population (n = 122) are shown in Tables I–III and Supplementary Table SI.
Table I
Baseline demographic, clinical, and biological characteristics of the study population
[i] BMI – body mass index, CKD – chronic kidney disease, CRP – C-reactive protein, DBP – diastolic blood pressure, eGFR – estimated glomerular filtration rate, HDL – high-density lipoprotein, LDL – low-density lipoprotein, NT-proBNP – N-terminal pro-brain natriuretic peptide, SBP – systolic blood pressure. CKD was defined according to the Kidney Disease Improving Global Outcomes guidelines [22].
Table II
Echocardiographic and bioimpedance characteristics of the study population
[i] AFO – absolute fluid overload, ECW – extracellular water, ICW – intracellular water, LA – left atrial, LVEF – left ventricular ejection fraction, LV EDD – LV end diastolic diameter, LV EDVi – left ventricular end diastolic volume index, LV ESD – LV end systolic diameter, LV ESVi – left ventricular end systolic volume index, RFO – relative fluid overload, TBW – total body water.
Table III
Univariable and multivariable associates of B-lines (log transformed) in the study population
[i] CRP – C-reactive protein, ECW – extracellular water, eGFR – estimated glomerular filtration rate, ICW – intracellular water, LA – left atrial, LVEF – left ventricular ejection fraction, LV ESVi – left ventricular end systolic volume index, NT-proBNP – N-terminal pro-brain natriuretic peptide, TBW – total body water.
The investigation conforms with the principles outlined in the Declaration of Helsinki [20]. The study was approved by the Research Ethics Committee of the “Grigore T. Popa” University of Medicine and Pharmacy Iasi, and all included patients signed an informed consent form. The trial was registered at ClinicalTrials.gov, NCT02764073.
Demographic and clinical parameters
The following demographic parameters were recorded at baseline: age, gender, weight, height, comorbidities (diabetes, coronary artery disease, hypertension, atrial fibrillation, chronic kidney disease (CKD)), and smoking status. Arterial blood pressure was determined in the morning in all patients by a physician by three consecutive measurements, after a 15-minute resting period, with the mean values calculated for systolic (SBP) and diastolic blood pressure (DBP). We used an automatic BP measuring device certified by the Association for the Advancement of Medical Instrumentation, European Society of Hypertension, and British Society of Hypertension – model OMRON M6, with an adjustable cuff for arm circumferences from 24 to 42 cm, respecting the current guideline recommendations [21]. Hypertension was defined as a SBP of at least 140 mm Hg and/or diastolic BP of at least 90 mm Hg or previously diagnosed hypertension under treatment during the previous 2 weeks, regardless of BP values. We considered that a patient had coronary artery disease if in the medical records there was a diagnosis of coronary artery/heart disease, angina or angina pectoris, or myocardial infarction. Chronic kidney disease was defined according to the Kidney Disease Improving Global Outcomes guidelines [22].
We also evaluated diuresis, the presence of peripheral oedema (slight pitting of at least 2 mm depth with no visible distortion) [23], the New York Heart Association (NYHA) functional class, and the medication.
Biochemical analysis
All blood samples were obtained from patients in the morning, after 12 h of fasting, for measurement of serum creatinine, haemoglobin, glucose, total cholesterol, triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL) cholesterol, C-reactive protein (CRP), uric acid, and sodium levels. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [24]. All laboratory tests were performed by standard procedures with certified methods on the day of the lung ultrasonography and BIS assessments.
We also stored serum at –80°C for subsequent analyses. N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) in these samples was analysed using the Elabscience® ELISA Kit, an electro-chemiluminescence ‘sandwich’ immunoassay based on antibodies against Human NT-proBNP.
Echocardiographic assessment
Echocardiographic measurements were performed before (the same day or maximum 1 day before) LUS and BIS evaluations by two trained echocardiographers according to the recommendations of the American Society of Echocardiography [25].
B-line assessment
Lung ultrasonography was performed with patients in the supine position, for a total of 28 sites per complete examination, as previously described [26]. At every scanning site, B-lines could be counted from 0 (no B-lines) to 10 (complete white screen) (Supplementary Figure S1). The sum of the B-lines gave a score representing the extent of extravascular lung water. Two trained physicians blinded to the echocardiography assessment performed all measurements.
Bioimpedance analysis
The hydration state and the body composition were assessed immediately after the LUS evaluation using a portable whole-body BIS device (BCM – Fresenius Medical Care D GmbH [27]). By attaching electrodes to the patient’s forearm and ipsilateral ankle, this device measures the impedance spectroscopy at 50 frequencies. The extracellular water (ECW), intracellular water (ICW), and TBW were determined as previously described [28].
Outcome
The main outcome was all-cause mortality. Death was confirmed through patient follow-up phone calls, the hospital’s electronic medical records, or the social security death index.
Statistical analysis
Variables were expressed as median with interquartile range (IQR), mean ± standard deviation (SD), or as percentage of frequency, as appropriate. For the categorical variables, the between-group comparisons were performed using the χ2 test, and for the continuous variables using the Mann-Whitney U test or the independent T-test, as appropriate. The Shapiro-Wilk test was used for assessing the normality of the distribution and logarithmic conversion was performed for non-normally distributed variables, including B-lines number, glucose, HDL and LDL-cholesterol, triglycerides, CRP, and uric acid levels.
Pearson’s correlation coefficient was used to determine correlations between variables. Backward stepwise multivariable regression analysis including all univariate associates (p < 0.05) was used to assess the predictors for B-lines.
Time-to-event analysis of all-cause mortality was performed using Kaplan-Meier cumulative survival plots and Cox proportional hazards model, including adjustment for potential confounding factors. In model 1, we adjusted for clinical risk factors: age, sex, and diabetes. In model 2, we adjusted for biochemical and echocardiographic (LVEF) risk factors: CRP, eGFR, NT-proBNP, and LVEF. In model 3, we adjusted for all the variables used in previous two models. We also made an additional model (model 4) in which we adjusted also for ECW (for the B-line analysis) or B-lines (for the ECW analysis).
To determine the optimal cut-off point for the number of B-lines and ECW as predictors of all-cause mortality, the relationship between the number of B-lines and ECW and the outcome was analysed using the Martingale residuals in Cox’s proportional hazard regression analysis [29]. We found that the categorisation of the number of B-lines and ECW into two categories (using 15 B-lines and 18.3 l as cut-offs for the number of B-line and ECW, respectively) was the best approach for modelling these relationships. Analyses were performed with B-lines and ECW as a categorical (based on the cut-offs mentioned above) and also as a continuous variable. Data are presented in the form of Hazard ratios and 95% confidence intervals. We tested the proportional hazards assumptions using the Schoenfeld residuals, and when needed we performed bootstrapping validation in order to avoid the problem of over fitting due to the low number of death events.
All analyses were performed using Stata SE software, version 13 (StatCorp, College Station, TX, USA) and the R (version 3.6.1) package for statistical analysis (Foundation for Statistical Computing, Vienna, Austria). A p-value < 0.05 was considered to be statistically significant.
Results
Characteristics of the study population
Our study included 122 patients (67.2% males) with a mean age of 67.2 years. The most frequent cause of HF was dilated cardiomyopathy – 47 (43.1%) patients. However, the exact cause of HF was unknown in 25 (20.5%) patients. More than half had at least NYHA class III; the median number of B-lines was 16. Other characteristics of the population are presented in Tables I and II and Supplementary Table SI.
As a first step we divided the study patients according to the number of B-lines (Group 1 B-lines below 15 and Group 2 B-lines with at least 15 B-lines). As expected, patients in Group 1 B-lines had a lower prevalence of oedema and less severe NYHA class; they also showed better renal function (as assessed by eGFR) and lower CRP, NT-proBNP, and uric acid levels than patients from Group 2 B-lines (Table I). Furthermore, these patients had lower left atrial and left ventricular volumes and a better LVEF than patients from Group 2 B-lines (Table II). Bioimpedance spectroscopy fluid status assessment showed that although there was no significant difference between these two groups in TBW, patients from Group 1 B-lines had lower ECW and higher ICW volumes (Table II).
Correlations of lung congestion with demographic, clinical, biological, and bioimpedance parameters
In univariable analysis, we identified eGFR, CRP, serum sodium, NT-proBNP, and different clinical, echocardiographic, and BIS derived characteristics as significant correlates for the number of B-lines. In multivariable linear regression analysis, including all the univariable predictors of lung congestion, only NYHA class, ECW, eGFR, and LVEF levels maintained an independent association (R2 of the model = 0.42) with the number of B-lines (Table III).
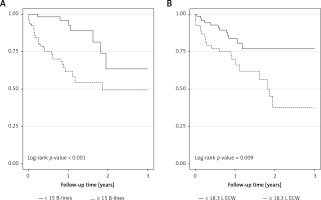
All-cause mortality according to the number of B-lines and extracellular water volume
During the follow-up (mean 12.5 months, median 11.2 months), 33 (27.1%) patients died. There were seven deaths in the group of patients with the number of B-lines below 15, while in the other group there were 26 deaths (there were 12 deaths in the group of patients with below 18.3 l of ECW and 21 deaths in the group with at least 18.3 l of ECW). Kaplan-Meier curves show significantly higher all-cause mortality in HF patients with at least 15 B-lines (Figure 1 A) or 18.3 l ECW volume (Figure 1 B). In unadjusted Cox regression, patients from Group 2 B-lines had a HR of 3.96 (95% CI: 1.78–8.79), while those in Group 2 ECW had a HR for death of 2.47 (95% CI: 1.23–4.98) for the outcome of interest (Table IV). In multivariable Cox analysis, a number of B-lines of at least 15 remained significantly associated with all-cause mortality, independently of different clinical, echocardiographic, biological, or BIS-derived fluid characteristics (Table IV). However, an 18.3 l of ECW volume remained significantly associated with the outcome after adjustments for clinical, echocardiographic, or biological risk factors (Table IV, Models 1 and 2) but lost this significance after adjustment for all the aforementioned risk factors (Table IV, Model 3) or for lung congestion (Table IV, Model 4).
Table IV
Lung congestion and extracellular water as predictors of all-cause mortality (using the B-lines and extracellular water groups)
| Variable | B-lines | ECW | ||
|---|---|---|---|---|
| HR* | 95% CI | HR* | 95% CI | |
| Unadjusted | 3.96 | 1.78–8.79 | 2.47 | 1.23–4.98 |
| Adjusted: | ||||
| Model 1 | 4.88 | 1.66–14.34 | 2.19 | 1.07–4.48 |
| Model 2 | 3.58 | 1.55–8.29 | 2.24 | 1.09–4.59 |
| Model 3 | 3.87 | 1.16–12.91 | 1.99 | 0.94–4.18 |
| Model 4 | 3.84 | 1.12–13.09 | 1.62 | 0.75–3.51 |
CI – confidence interval, ECW – extracellular water, HR – hazard ratio. Model 1: age, sex, diabetes. Model 2: C-reactive protein, estimated glomerular filtration rate, left ventricular ejection fraction, N-terminal pro-brain natriuretic peptide. Model 3: variables in model 1 + variables in model 2. Model 4: Model 3 + ECW (for the B-lines groups) or Model 3 + B-lines (for the ECW groups).
Using the number of B-lines and ECW as continuous variables, we found in univariate Cox analysis that both variables were associated with an elevated risk of death (HR = 2.04, 95% CI: 1.36–3.08 and HR = 1.16, 95% CI: 1.03–1.29 for each increment of 1 SD in log number of B-lines and 1 l in ECW volume, respectively). After adjustment, the number of B-lines remained significantly associated with the outcome in all the multivariable Cox models, while ECW lost the significance for this relationship after adjustment for biological characteristics and LVEF (Supplementary Table SII).
Discussion
This study shows for the first time that, in HF patients with a reduced LVEF, lung congestion is dependent on eGFR, LVEF, and ECW volume, as assessed by BIS. We also confirm the increased risk of death associated with pulmonary congestion, but we show that this association is independent of cardiac and renal function or body fluid compartments.
Congestion is the main feature and reason for hospital admission of HF patients. However, the physiopathological transition from haemodynamic congestion (increased left ventricular filling pressures) to lung congestion (increased extravascular lung water) and finally to clinical congestion (signs and symptoms of congestion) is complex and still incompletely understood. Extravascular lung water, a relatively small component of body fluids compartments, is usually related to increased left ventricular filling pressures and total body fluids. Some studies support the idea that pulmonary congestion is related more to fluid redistribution than to total body fluid accumulation. In the IMPACT-HP trial the authors demonstrated that the degree of weight loss was not associated with clinical improvement [30]. Similarly, in the EVEREST study the weight reduction obtained with tolvaptan was not associated with improvements in global clinical status [31]. Our study confirms this hypothesis and adds new data into this puzzle. We show that although there is no difference in TBW between the patients with below and those with at least 15 B-lines, there are significantly higher ECW volumes in patients from the latter group. Furthermore, in the multivariable regression model, ECW is one of the variables that remained independently associated with lung congestion.
Another important piece in the puzzle is the relationship between eGFR, as a surrogate of renal function, and the degree of pulmonary congestion in HF patients. We show an inverse and independent association between eGFR and the number of B-lines. There is a known bidirectional lung-kidney crosstalk commonly present in health and specific pathological states [32]. As seen in acute kidney injury or CKD, there are elevations in the levels of different mediators that could alter pulmonary vascular permeability [32] and as such increase the risk of pulmonary congestion. However, also pulmonary congestion with impaired gas exchange could lead to decreased renal blood flow and alterations in eGFR through stimulation of adrenergic nerves and disturbances in nitric oxide metabolism [33]. Although in this relationship the primum movens is not known, our results clinically validate the increased risk of pulmonary congestion related to worse renal function in HF patients with reduced LVEF.
It is now well established that LUS, through the assessment of B-lines, is a practical diagnostical and prognostic tool in acute and chronic HF patients [12]. Findings from studies performed in chronic HF patients suggest that those with pulmonary congestion are at an increased risk of hospitalisation or death [13, 34–36]. With a fourfold increase in the risk for all-cause mortality, our findings are consistent with these studies; however, by comparison with the aforementioned studies, although the authors adjusted the results for demographic [34–36], clinical [13, 35], or cardiac characteristics [13, 34], our study is the only one that combines demographical clinical, cardiac, biological, and, most importantly, BIS-derived fluid status confounders into the survival analysis. The fact that the number of B-lines is associated with all-cause mortality even after all these adjustments reinforces the important role played by pulmonary congestion in predicting adverse outcomes in HF patients. Our cut-off of 15 B-lines, derived from statistical analysis, is the same as the clinical cut-off proposed and used in previous studies [12, 13].
Bioimpedance spectroscopy has shown promising results, especially in dialysis patients [18], in HF patients its use being limited to only two diagnostic studies performed in an acute setting with no follow-up outcomes [37, 38]. Although our study shows that an ECW higher than 18.3 l is associated with death in the initial multivariable Cox models, after different adjustments, including also pulmonary congestion, this association becomes non-significant. We obtained similar findings in a previous study performed in dialysis patients. In the final model, the number of B-lines, and not the BIS derived fluid status parameter, was associated with all-cause mortality [39].
This study supports previous findings regarding the impact of lung congestion on all-cause mortality in HF patients, but at the same time refines this association. We used a holistic approach in our analysis, trying to underline the complex relationship between the lung, heart, and kidney and its effect on survival in this type of patient.
Our study has some limitations that must be addressed. We included a relatively small sample size of selected HF patients from a single centre with a relatively low number of mortality events, but we used different statistical approaches to overcome these shortcomings. Furthermore, we are not able to completely exclude the existence of residual confounding in our regression models. These factors could limit the generalisability of the results obtained in this study. However, our findings are particularly encouraging because they emphasise the complex pathways that link lung congestion with cardiac and renal function and body fluid compartments. Ideally, these results should be validated in other HF populations, including larger samples. In addition, we could not specify the cause of death, and the data about nonfatal events were not collected.
In conclusion, we show for the first time in HF patients that pulmonary congestion, as assessed by lung ultrasonography, is associated with the severity of NYHA class, LVEF, eGFR, and ECW, and it identifies those at increased risk of death. Further studies are required to determine whether such early warnings, such as pulmonary or systemic fluid congestion, may prevent the clinical progression of disease and HF hospitalisation.